What is the collision theory in chemistry?
- According to the kinetic theory of matter, particles of matter are in continuous motion and constantly in collision with each other.
- For a reaction to occur, the particles of the reactants (atoms, molecules or ions) must touch each other through collision for bond breaking and bond formation to form the products.
- However, not all collisions will result in a reaction to form the products.
- According to the collision theory, only those collisions which
(a) achieve a minimum amount of energy, called activation energy, and
(b) with the correct orientation,
will result in reaction.
These types of collisions are known as effective collisions. - For particles that collide with energy less than the activation energy needed for reaction or with the wrong orientation, they simply bounce apart without reacting. These collisions are known as ineffective collisions.
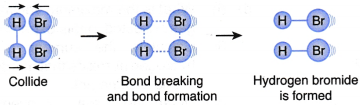
- An example to illustrate the collision theory
(a) (i) Hydrogen gas reacts with bromine gas to form hydrogen bromide gas
(ii) The chemical equation for the reaction is H2(g) + Br2(g) → 2HBr(g)
(b) Table illustrates three different situations that may happen during the collisions of hydrogen molecules with bromine molecules.
People also ask
- How does the collision theory affect the rate of reaction?
- What is the rate of the reaction?
- How do you calculate the reaction rate?
- What factors affect the rate of a reaction?
- How does the surface area affect the rate of reaction?
- Explain the effect of concentration on the rate of reaction?
- How does the temperature affect the rate of a chemical reaction?
- What is the effect of a catalyst on the rate of a reaction?
| Situations | Will it result in a reaction? |
I. A hydrogen molecule and a bromine molecule collide with the correct orientation but the energy of the colliding particles is less than the activation energy, as shown in Figure.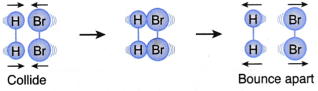 | This collision will not result in a reaction (Ineffective collision) |
II. A hydrogen molecule and a bromine molecule collide with their total energy greater than or equal to the activation energy but with the wrong orientation, as shown in Figure.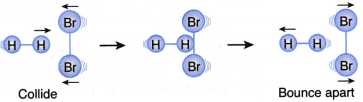 | This collision will not result in a reaction (Ineffective collision) |
III. A hydrogen molecule and a bromine molecule collide with their total energy greater than or equal to the activation energy and with the correct orientation, as shown in Figure. | An effective collision occurs. |
What is Activation Energy?
Activation energy:
- The meaning of activation energy can be visualised by sketching the energy profile diagrams, as shown in Figure.
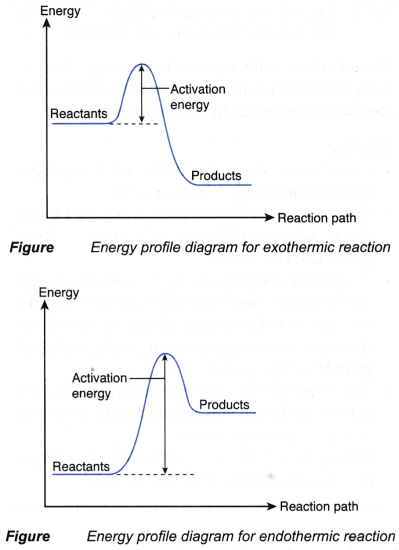
- The difference in energy between the energy of the reactants and the energy at the peak of the curve is the activation energy.
- Activation energy is the energy barrier that must be overcome by the colliding particles of the reactants so that the reaction can occur.
What is the relationship between activation energy and collision theory?
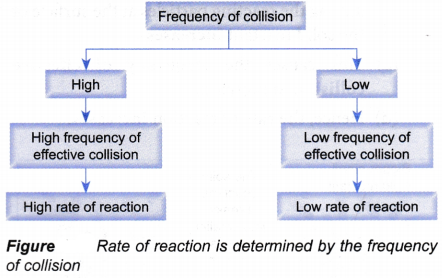
Relationship between the frequency of collision and the activation energy with the rate of reaction:
- According to the collision theory, the rate of a reaction is determined by the
(a) frequency of collision
(b) magnitude of activation energy - Frequency of collision
(a) If the frequency of collision for a reaction is high, then the frequency of effective collision that can result in a reaction is also high.
(b) Hence, the rate of reaction is high, and vice versa
(c) Figure illustrates this relationship.
- Magnitude of activation energy
(a) The values of are different for different reactions.
(b) If the activation energy of a reaction is high, fewer number of colliding particles are able to achieve this high energy. Hence, the frequency of effective collision is low. As a result, the rate of reaction is low.
(c) Conversely, if the activation energy of a reaction is low, the rate of reaction is high. - The effect of the size of a solid reactant/surface area, concentration, pressure, temperature and catalyst on the rate of reaction can be explained using the collision theory.