How does the collision theory affect the rate of reaction?
Explaining the effect of size of a solid reactant/surface area on the rate of reaction using collision theory
- When the size of a fixed mass of a solid reactant decreases, the rate of reaction increases.
- This can be explained using the collision theory, as below:
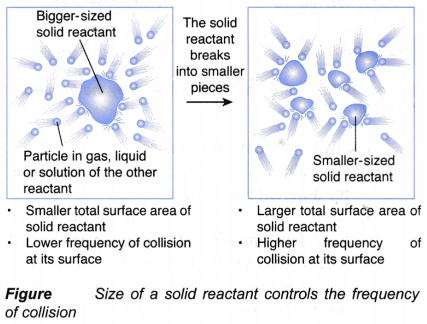
(a) When the size of a fixed mass of a solid reactant becomes smaller, the total surface area exposed to collision with the particles of the other reactants increases, as shown in Figure.
(b) As a result, the frequency of collision among the reacting particles at the surface of the solid reactant increases.
(c) This causes the frequency of effective collision to increase.
(d) Hence, the rate of reaction increases.
Example: Two sets of experiments are carried out as shown below.
Set I: 1.0 g of granulated zinc is added to 20 cm3 of 0.5 mol dm-3 sulphuric acid at 27.0°C.
Set II: 1.0 g of zinc powder is added to 20 cm3 of 0.5 mol dm-3 sulphuric acid at 27.0°C.
The initial rate of liberation of hydrogen gas in set II is higher than that in set I. Explain the difference in the rate of reaction using the collision theory.
Solution:
- Particle size of zinc powder in set II is smaller than that of granulated zinc in set I.
- Thus, the total exposed surface area of 1.0 g of zinc powder in set II is larger than that of 1.0 g of granulated zinc in set I.
- As a result, the frequency of collision between the hydrogen ions from sulphuric acid and the zinc atoms at the surface of zinc powder in set II is higher than that occurring at the surface of granulated zinc in set I.
- This causes the frequency of effective collision between hydrogen ions and zinc atoms in set II to be higher than that in set I.
- Hence, the initial rate of liberation of hydrogen gas in set II is higher than that in set I.
People also ask
- What is the collision theory in chemistry?
- What is the rate of the reaction?
- How do you calculate the reaction rate?
- What factors affect the rate of a reaction?
- How does the surface area affect the rate of reaction?
- Explain the effect of concentration on the rate of reaction?
- How does the temperature affect the rate of a chemical reaction?
- What is the effect of a catalyst on the rate of a reaction?
Explaining the effect of concentration on the rate of reaction using collision theory
- When the concentration of a reactant increases, the rate of reaction increases.
- This can be explained using the collision theory, as below:
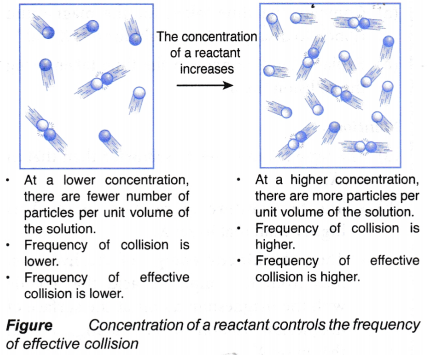
(a) When the concentration of the solution of a reactant increases, the number of particles per unit volume in this solution also increases.
(b) With more particles per unit volume, the frequency of collision among the reacting particles increases, as shown in Figure.
(c) Hence, the frequency of effective collision increases.
(d) As a result, the rate of reaction increases.
Example: Two experiments are carried out as shown below:
| Experiment | Reacting conditions |
| I | 50 cm3 of 0.20 mol dm-3 sodium thiosulphate solution + 5 cm3 of 1.0 mol dm-3 hydrochloric acid |
| II | 50 cm3 of 0.12 mol dm3 sodium thiosulphate solution + 5 cm3 of 1.0 mol dm-3 hydrochloric acid |
(a) Which of the two experiments shows a higher rate of reaction?
(b) Explain the difference in (a) using collision theory.
Solution:
(a) Rate of reaction of Experiment I is higher than that of Experiment II
(b)
- The concentration of sodium thiosulphate solution in Experiment I is higher than that in Experiment II.
- So, the number of thiosulphate ions, S2O32- in one dm3 of sodium thiosulphate solution in Experiment I is higher than that in Experiment II.
- This causes the frequency of collision between the reacting particles (thiosulphate ions and hydrogen ions) in
- Experiment I to be higher than that in Experiment II.
- Hence, the frequency of effective collision in Experiment I is higher than that in Experiment II that results iii a higher rate of reaction in Experiment I.
Explaining the effect of pressure of gaseous reactants on the rate of reaction using collison theory
- When the pressure of a reaction involving gaseous reactants increases, the rate of reaction increases.
- This can be explained using the collision theory, as below:
(a) When particles of gaseous reactants are compressed to occupy a smaller volume, the pressure of the gaseous reactants increases, as shown in Figure.
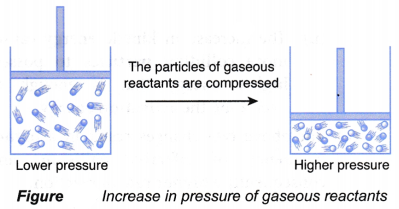
(b) Thus, the number of gas particles per unit volume increases.
(c) With more gas particles per unit volume, the frequency of collision among the reacting particles increases.
(d) Hence, the frequency of effective collision increases, which results in a higher rate of reaction.
Explaining the effect of temperature on the rate of reaction using collision theory
- When the temperature of a reaction increases, the rate of reaction increases.
- This can be explained using the collision theory, as below.
(a) An increase in temperature will cause the kinetic energy of the reacting particles to increase.
(b) As a result, it leads to the following two changes:
(i) The reacting particles move faster and collide more often with one another. Hence, the frequency of collision increases, as shown in Figure.
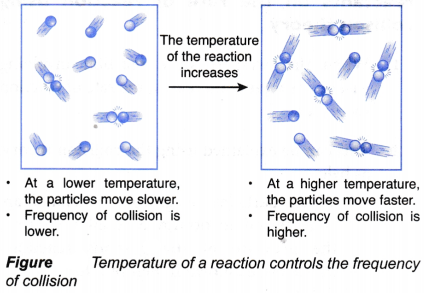
(ii) The increase in kinetic energy causes more colliding particles to possess higher energy that is enough to overcome the activation energy.
(c) The above two changes result in a higher frequency of effective collision, and consequently a higher rate of reaction.
Example: Two sets of experiments are carried out as shown below.
Set X: 2 cm of magnesium ribbon is added to 50 cm3 of 1 mol dm-3 sulphuric acid at room temperature.
Set Y: 2 cm of magnesium ribbon is added to 50 cm3 of 1 mol dm-3 hot sulphuric acid at 80°C.
(a) Compare the time taken for the magnesium ribbon to disappear from sight for sets X and Y.
(b) Explain the difference in the time taken using the collision theory.
Solution:
- The time taken in set Y is shorter than that in set X.
- The temperature of reaction in set Y is higher than that in set X.
Thus, the kinetic energy of the hydrogen ions from the sulphuric acid that collide with the magnesium atoms at the surface of magnesium ribbon in set Y is higher than that in set X. - As a result, it leads to the following two changes:
(i) The hydrogen ions move faster and collide more often with the magnesium atoms in set Y compared to set X. The frequency of collision increases in set Y.
(ii) More collisions between hydrogen ions and magnesium atoms in set Y possess enough energy to overcome the activation energy compared to set X. - The above two changes result in a higher frequency of effective collision, and consequently a higher rate of reaction in set Y compared to set X.
- Hence, the time taken for set Y is shorter than that in set X.
Explaining the effect of catalyst on the rate of reaction using collision theory
- When a positive catalyst is used in a reaction, the rate of reaction increases.
- This can be explained using the collision theory, as below:
(a) When a positive catalyst is used in a chemical reaction, it enables the reaction to occur through an alternative path which requires a lower activation energy, as illustrated in the energy profile diagrams in Figure.
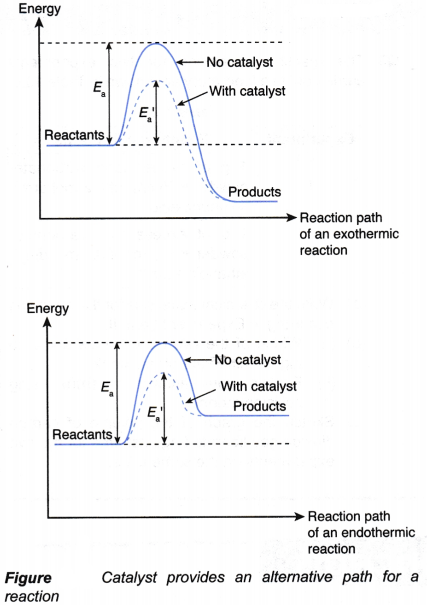
(b) As a result, more colliding particles are able to overcome the lower activation energy.
(c) Hence, the frequency of effective collision increases, and consequently this results in a higher rate of reaction.
Example: Two sets of experiments are carried out as shown below.
Set Q: 2 g of granulated zinc is added to 50 cm3 of 0.2 mol dm-3 hydrochloric acid at room conditions.
Set R: 2 g of granulated zinc is added to a mixture of 50 cm3 of 0.2 mol dm-3 hydrochloric acid and 2 cm3 of 1 mol dm-3 copper(II) sulphate solution at room conditions.
Explain why initial rate of set R is higher than that of set Q using the collision theory.
Solution:
- Copper(II) sulphate solution in set R acts as a positive catalyst, whereas no catalyst is used in set Q.
- The presence of copper(II) sulphate in set R enables the reaction between zinc and hydrogen ions from hydrochloric acid to occur through an alternative path which requires a lower activation energy.
- As a result, more collisions between the hydrogen ions and zinc atoms at the surface of granulated zinc are able to overcome the lower activation energy in set R.
- Hence, the frequency of effective collision increases. Consequently, this results in a higher rate of reaction for set R.
Appreciating scientist’s contributions
- Scientists always practice problem-solving attitudes.
- The patience, hard work and perseverance of scientists in doing their research and development had led to the discovery of the factors affecting the rate of reaction.
- With this knowledge, we can control the rate of reaction in many activities in our daily life.
- We must be thankful to those scientists for their contributions to improve the quality of life.
