Classification of Salts
What is a salt?
A salt is formed in a neutralisation reaction between an acid and a base. Salt is a general term used for the substances that are formed when an acid and a base react with each other.
This reaction is called a neutralization reaction.
Acid + base → salt + water
A salt is an ionic compound consisting of a cation such as a metal ion or an ammonium ion from a base and an anion from an acid.
Example:
The reaction between vinegar and baking soda is a neutralization reaction. Vinegar, as we already know, contains an acid and baking soda contains a base. When vinegar reacts with baking soda, a salt is formed. Water and carbon dioxide gas are also the products of this reaction.
Similarly, common table salt is formed by the reaction of hydrochloric acid (HCl) with sodium hydroxide (NaOH).

HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
Hence, a salt can be defined as follows.
A salt is a compound formed when the hydrogen ion in an acid is replaced by a metal ion or an ammonium ion.
People also ask
- General Properties of Salts
- Uses of different salts in daily life
- Preparation of Salts
- Describe the preparation of soluble and insoluble salts
- Qualitative Analysis of Salts
- Action of Heat on Salts
- Test for Cations and Anions in Aqueous Solutions
- Constructing ionic equations using the continuous variation method
- What is stoichiometry and why is it used in chemistry?
Table: Examples of salts from different acids
| Acid | Salt | |||
| Hydrochloric acid | HCl | Chloride salts | Sodium chloride Ammonium chloride | NaCl NH3Cl |
| Nitric acid | HNO3 | Nitrate salts | Potassium nitrate Aluminium nitrate | KNO3 Al(NO3)3 |
| Sulphuric acid | H2SO4 | Sulphate salts | Ammonium sulphate Magnesium sulphate | (NH4)2SO4 MgSO4 |
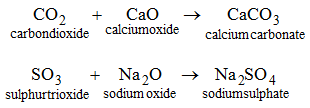
| Carbonic acid | H2CO3 | Carbonate salts | Iron(ll) carbonate Calcium carbonate | FeCO3 CaCO3 |
| Phosphoric acid | H3PO4 | Phosphate salts | Iron(lll) phosphate Ammonium phosphate | FePO4 (NH4)3PO4 |
| Ethanoic acid | CH3COOH | Ethanoate salts | Lead(ll) ethanoate Copper(ll) ethanoate | Pb(CH3COO)2 Cu(CH3COO)2 |
Salts can be acidic, basic, or neutral. Acidic salts are formed when a strong acid reacts with a weak base. Basic salts are formed when a strong base reacts with a weak acid. When a strong acid reacts with a strong base, neutral salts are formed.

Types of Salts:
The different types of salts are: normal salt, acid salt, basic salt and double salt.
1. Normal salt : A salt that does not contain any replaceable hydrogen atoms or hydroxyl groups is called normal salt.
Examples :
Na2SO4 obtained in the reaction between H2SO4 and NaOH is a normal salt because it is formed by the complete replacement of both the H atoms of H2SO4,
Similarly, calcium su1phate (CaSO4), sodium phosphate (Na3PO4) and potassium phosphate (K3PO4) are also normal salts.
2. Acid salt : When a polybasic acid is not completely neutralized by a base, the salt produced will contain replaceable hydrogen atoms. Hence, it may further take part in the reaction with the base as an acid. Such a salt is called an acid salt. For example, the salt NaHSO4 produced in the reaction between NaOH and H2SO4 is an acid salt because it is capable of further reaction with the base NaOH to produce the normal salt Na2SO4.
H2SO4 + NaOH → NaHSO4 + H2O
NaHSO4 + NaOH → Na2SO4 + H2O
Thus, an acid salt may be defined as follows.
A salt that contains replaceable hydrogen atoms is called an acid salt.
Examples :
NaHSO4, NaH2PO4 and Na2HPO4 are examples of acid salts.
3. Basic salt : When a polyacidic base reacts with lesser amount of acid than is necessary for complete neutralization, the salt produced contain hydroxyl group(s) (OH) also. Such a salt is called a basic salt.
Examples :
1 mole of Pb(OH)2 requires 2 moles of HCl for complete neutralization. But when 1 mole of Pb(OH)2 is made to react with 1 mole of HCl, some Pb(OH)2 is left unreacted. The salt produced is not PbCl2, but Pb(OH)Cl.
![]()
Similarly, when one mole of Bi(OH)3 is reacted with 1 mole of HNO3, the salt Bi(OH)2NO3 is formed.
Bi(OH)3 + HNO3 → Bi(OH)2NO3 + H2O
Salts like Pb(OH)Cl and Bi(OH)2NO3 contain the OH group. These salts are called basic salts, because they can further react with the acids to form H2O and the corresponding normal salts.
Pb(OH)Cl + HCl → PbCl2 +H2O
Bi(OH)2NO3 + HNO3 → Bi(OH)(NO3)2 + H2O
Bi(OH)(NO3)2 + HNO3 → Bi(NO3)3 + H2O
Thus, a basic salt is formed when a poly acidic base reacts with a lesser amount of an acid than is necessary for the formation of a normal salt.
4. Double salt : In a double salt, there are two different negative ions and/or positive ions. For example, the mineral dolomite, CaCO3·MgCO3, contains both Ca2+ and Mg2+ ions. Hence, it is a double salt. Potash alum, K2SO4·Al2(SO4)3.24H2O, also is a double salt.
Double salts exist only in the solid state. When dissolved in water, they break up into a mixture of two separate salts. For example, when potash alum is dissolved in water, it breaks up as follows.
Solubility of salts in water
- Salts are ionic compounds and they can dissolve in water. However, through experiments conducted by chemists not all salts are found to be soluble in water.
- For example, sodium chloride, NaCl dissolves readily in water, but silver chloride, AgCl cannot dissolve in water.
- Table lists out some common soluble and insoluble salts.
Table: Solubility of salts in water
| Type of salt | Solubility in water |
| Ammonium salts | All are soluble. |
| Sodium and potassium salts | All are soluble. |
| Ethanoate salts | All are soluble. |
| Nitrate salts | All are soluble. |
| Chloride salts | All are soluble except AgCl, HgCI and PbCl2 |
| Sulphate salts | All are soluble except PbSO4, CaSO4, BaSO4, and Ag2SO4 |
| Carbonate salts | All are insoluble except Na2CO3, K2CO3, and (NH4)2CO3 |
| Lead(ll) salts | All are insoluble except Pb(NO3)2 and Pb(CH3COO)2 |
Activity
Aim: To demonstrate a neutralization reaction
Materials needed: Sodium hydroxide, dilute hydrochloric acid, and phenolphthalein
Method:
Take 30 ml of dilute sodium hydroxide solution in a conical flask and add a drop of phenolphthalein to it. Now pour hydrochloric acid to it dropwise, usjng a dropper till there is some change in the colour of the solution.
Observation: The solution turns pink when phenolphthalein is added to sodium hydroxide. The solution turns colourless when the entire sodium hydroxide has reacted. On testing the colourless solution with litmus paper, there is no change either with red litmus or blue litmus paper.
Conclusion: There is no change in the colour of the litmus paper indicating that the solution has turned neutral.
