Explain Classification of Elements
Elements
An element is a substance which cannot be split up into two or more simpler substances by the usual chemical methods of applying heat, light or electric energy.
An element cannot be split up into two (or more) simpler substances because it is made of only one kind of atoms.
Ex. Hydrogen is an element because it cannot be split up into two or more simpler substances by the usual methods of carrying out chemical reactions by applying heat, light or electricity.
| Element | Symbol |
| Aluminium | Al |
| Arsenic | As |
| Barium | Ba |
| Bromine | Br |
| Cadmium | Cd |
| Calcium | Ca |
| Chlorine | Cl |
| Chromium | Cr |
| Cobalt | Co |
| Fluorine | F |
| Hydrogen | H |
| Iodine | I |
| Magnesium | Mg |
| Manganese | Mn |
| Nitrogen | N |
| Oxygen | O |
| Phosphorus | P |
| Sulphur | S |
| Uranium | U |
| Zinc | Zn |
| (symbols from latin names) | |
| Antimony (stibium) | Sb |
| Copper (Cuprum) | Cu |
| Gold (Aurum) | Au |
| Iron (Ferrum) | Fe |
| Lead (Plumbum) | Pb |
| Mercury (Hydrogyrum) | Hg |
| Potassium (Kalium) | K |
| Silver (Argentum) | Ag |
| Sodium (Natrium) | Na |
| Tin (Stannum) | Sn |
All the Elements can be divided into three groups.
- Metal
- Non-metal
- Metalloid
1. Metals :
A metal is an element that is malleable and ductile, and conducts electricity. All the metals are solids except one metal mercury, which is a liquid.
Ex. Iron, Copper, Aluminium, Zinc.
Properties of metals
- Metals are malleable : This means that metals can be beaten into thin sheets with a hammer (without breaking).
Ex. Aluminium metal is quite malleable and can be converted into thin sheets called aluminium foils. Aluminium foils are used for packing food items like biscuits, chocolates, medicines, cigarettes, etc. - Metals are ductile : This means that metals can be drawn (or stretched) into thin wires. All the metals are not equally ductile. Some are more ductile than the other.
Ex. Copper and aluminium metals are also very ductile and can be drawn into thin wires which are used in electrical wiring. - Metals are good conductors of heat and electricity : This means that metals allow heat and electricity to pass through them easily. Silver metal is the best conductor of heat. It has the highest thermal conductivity.
Ex. The cooking utensils and water boilers, etc., are usually made of copper or aluminium metals because they are very good conductors of heat.
Ex. The electric wires are made of copper and aluminium metals because they are very good conductors of electricity. - Metals are lustrous (or shiny), and can be polished : The property of a metal of having a shining surface is called metallic lustre (chamak). The shiny appearance of metals makes them useful in making jewellery and decoration pieces
Ex. Gold and silver are used for making jewellery because they are bright and shiny. The shiny surface of metals makes them good reflectors of light. Silver metal is an excellent reflector of light. - Metals are generally hard :
Most of the metals are hard. But all the metals are not equally hard. The hardness varies from metal to metal they can not cut with a knife. (except sodium and potassium which are soft metals).
Ex. Iron, copper, aluminium. - Metals are usually strong. They have high tensile strength : This means that metals can hold large weights without breaking.
Ex. Iron metal (in the form of steel) is very strong having a high tensile strength. Due to this iron metal is used in the construction of bridges, buildings, railway lines, girders, machines, vehicles and chains etc. - Metals are solids at the room temperature :
All the metals like iron, copper, aluminium, silver and gold, etc., are solids at the room temperature. Only one metal, mercury, is in liquid state at the room temperature. - Metals generally have high melting points and boiling points : This means that most of the metals melt and vaporise at high temperatures.
Ex. Iron is a metal having a high melting point of 1535ºC. This means that solid iron melts and turns into liquid iron on heating to a high temperature of 1535ºC. - Metals have high densities : This means that metals are heavy substances.
Ex. The density of iron metal is 7.8 g/cm3 which is quite high. - Metals are sonorous : This means that metals make a ringing sound when we strike them.
Ex. Plate type musical instruments like cymbals (manjira), and wires (or strings) for stringed musical instruments such as violin, guitar, sitar and tanpoora, etc. - Metals usually have a silver or grey colour : (except copper and gold). Copper has a reddish-brown colour whereas gold has a yellow colour.
- Metallic Bonding
The bonding which holds the metal atoms firmly together on account of force of attraction between metal ions and the mobile electron is called metallic bonding.
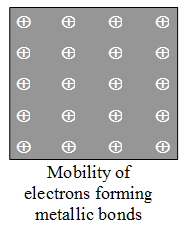 X-rays analysis of metal crystal has revealed that each atom in metal crystal is surrounded by 8 or 12 other metal atoms. In metal atoms, the valency electrons are few (1, 2, and 3) and thus, it is not possible for a metal atom to form 8 to 12 covalent bonds with neighbouring atoms. Thus, it was assumed that the atoms in metal crystal are bonded with each other with a special type of bonding known as metallic bonding. Drude in 1900 proposed the theory of metallic bonding which was later on modified by Lorentz. According to these authors, metals having 1, 2 or 3 electrons in outermost shells, being electropositive lose their electron readily because of low IE values to form free electrons and remainder portion of atom with a Kernel (core of stable nature) carrying positive charge. The free electrons are mobile in nature and move from the one Kernel to another which are closely packed in regular fashion throughout the crystal lattice. thus, the metal crystal is represented by an arrangement of positively charged Kernels in a sea of mobile electrons (Figure) shared by each Kernal to give metallic bonds. Ad the shared electrons are delocalized, the metallic bonds have neither direction nor saturation. There are two essential conditions for metallic bonding :
X-rays analysis of metal crystal has revealed that each atom in metal crystal is surrounded by 8 or 12 other metal atoms. In metal atoms, the valency electrons are few (1, 2, and 3) and thus, it is not possible for a metal atom to form 8 to 12 covalent bonds with neighbouring atoms. Thus, it was assumed that the atoms in metal crystal are bonded with each other with a special type of bonding known as metallic bonding. Drude in 1900 proposed the theory of metallic bonding which was later on modified by Lorentz. According to these authors, metals having 1, 2 or 3 electrons in outermost shells, being electropositive lose their electron readily because of low IE values to form free electrons and remainder portion of atom with a Kernel (core of stable nature) carrying positive charge. The free electrons are mobile in nature and move from the one Kernel to another which are closely packed in regular fashion throughout the crystal lattice. thus, the metal crystal is represented by an arrangement of positively charged Kernels in a sea of mobile electrons (Figure) shared by each Kernal to give metallic bonds. Ad the shared electrons are delocalized, the metallic bonds have neither direction nor saturation. There are two essential conditions for metallic bonding :- The metal atoms should have low ionization energy.
- There should be sufficient number of vacant orbitals.
The strength of metallic bonds increases with increase in :
(i) Number of valence electrons
(ii) Charge on the nucleus.
It is therefore, explained that alkali metals are soft and have low melting point, boiling point in comparison to transition metals which are hard and have high m.p., b.p. since, transition metals possess higher number of valence electrons as well as the higher charge on nucleus.
It is metallic bonding which explains the electrical and thermal conductance, metallic luster, malleability, ductility m.pt., b.pt., hardness in metals.
2. Non–Metals :
A non-metal is an element that is neither malleable nor ductile, and does not conduct electricity. All the non-metals are solids or gases, except bromine which is a liquid non-metal at room temperature.
Ex. Some of the examples of non-metals are : Carbon, Sulphur, Phosphorus, Hydrogen, Oxygen, Nitrogen, Chlorine, Bromine, Iodine, Helium, Neon, Argon, Krypton, and Xenon. Diamond and graphite are also non-metals.
Properties of non-metals
The physical properties of non-metals are just the opposite of the physical properties of metals.
- Non-metals are not malleable.
- Non-metals are brittle.
- Non-metals are not ductile. This means that non-metals cannot be drawn into wires. They are easily snapped on stretching.
- Non-metals are bad conductors of heat and electricity.
- Non-metals are not lustrous (not shiny). They are dull in appearance.
- Non-metals are generally soft
- Non-metals are not strong. They have low tensile strength.
- Non-metals may be solid, liquid or gases at the room temperature.
- Non-metals have comparatively low melting points and boiling points
- Non-metals have low densities.
- Non-metals are not sonorous.
- Non-metals have many different colours.
Comparison Among the Properties of metals and non-metals.
Metals | Non-Metals |
1. Metals are malleable and ductile. That is, metals can be hammered into thin sheets and drawn into thin wires. | 1. Non-metals are brittle. They are neither malleable nor ductile. |
2. Metals are good conductors of heat and electricity. | 2. Non-metals are bad conductors of heat and electricity (except diamond which is a good conductor of heat, and graphite which is a good conductor of electricity). |
3. Metals are lustrous (shiny) and can be polished. | 3. Non-metals are nonlustrous (dull) and cannot be polished (except iodine which is a lustrous non-metals). |
4. Metals are solids at room temperature (except mercury which is a liquid metal). | 4. Non-metals may be solid liquid or gases at the room temperature. |
5. Metals are strong and tough. They have high tensile strength. | 5. Non-metals are not strong. They have low tensile strength. |
6. Metals are sonorous. They make a ringing sound when struck. | 6.Non-metals are not sonorous. |
3. Metalloids :
The elements which show some properties of metals and some other properties of non-metals are called metalloids. Their properties are intermediate between the properties of metals and non-metals. Metalloids are also sometimes called semi-metals.
Ex. Boron (B), Silicon (Si), and Germanium (Ge).
