How do you Write a Chemical Equation?
All chemical reactions are represented by chemical equations. A chemical equation is a shorthand representation of a chemical reaction using the symbols and formulae of substance involved in the chemical reaction.
The symbols and formulae of the substances (elements or compounds) are arranged to show the reactants and products of a chemical reaction.
A chemical reaction occurs when starting substances react to produce new substances.
(a) Starting substances are called reactants.
(b) New substances formed are called products.
In an equation, the reactants are written at the left-hand side whereas the products are written at the right-hand side.
For example:
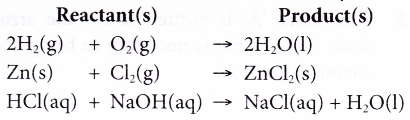
People also ask
- What is the Relative Atomic Mass and Relative Molecular Mass of an Element?
- What is One Mole and How many Particles are in a Mole?
- How do you Calculate the Molar Mass of a Substance?
- What is the Molar Volume of a Gas at STP?
- How do you know the Order of Elements in a Chemical Formula
- What is Empirical and Molecular Formula?
Constructing chemical equations
1. Based on the law of conservation of mass, matter can neither be created nor destroyed. This means that the numbers of atoms before and after a chemical reaction are the same. Therefore, a chemical equation must be balanced.
2. Table below shows how a chemical equation can be constructed.
Table: Constructing a chemical equation.
| Reaction: Iron filings react with copper(II) chloride to produce iron(III) chloride solution and copper. | |
| Step | Explanation and example |
| Identify the reactants, products and their formulae. | Reactants: Iron, Fe and copper(II) chloride, CuCl2 Products : Iron(III) chloride, FeCl3 and copper, Cu |
| Write the main part of the equation. | Fe + CuCl2 ⟶ FeCl3 + Cu Reactants Products |
| Determine the number of atoms of each element on both sides of the equation. | Left-hand side Right-hand side Fe atom : 1 Fe atom : 1 Cu atom : 1 Cu atom : 1 Cl atom : 2 Cl atom : 3 The numbers of atoms are not balanced. |
| Balance the equation by adjusting the coefficients. (Note: Coefficients are the numbers in front of the formulae.) | The Cl atoms are balanced. Fe + 3CuCl2 ⟶ 2FeCl3 + Cu As a result, the numbers of Fe atoms and Cu atoms are not balanced. The Fe atoms are then balanced. 2Fe + 3CuCI2 ⟶ 2FeCI3 + Cu Lastly, the Cu atoms are balanced. 2Fe + 3CuCI2 ⟶ 2FeCI3 + 3Cu |
| Check that the equation is balanced. | Left-hand side Right-hand side Fe atom : 2 Fe atom : 2 Cu atom: 3 Cu atom : 3 Cl atom : 6 Cl atom : 6 Now, the equation is balanced. |
| Put in the state symbol of each substance. | 2Fe(s) + 3CuCl2(aq) ⟶ 2FeCl3(aq) + 3Cu(s) |
3. The state symbols (s), (l) and (g) represent the solid, liquid and gaseous states respectively. The symbol (aq) represents an aqueous solution.
4. Sometimes the symbol ‘↑’ is used to indicate the release of a gas.
5. Sometimes ‘△’ is written above the arrow to show that heating is necessary to bring about a chemical reaction.
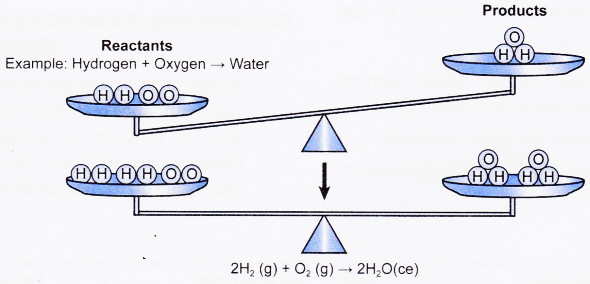
Rules for writing chemical equation:
Certain rules have to be followed while writing a chemical equation.
- The reactants taking part in the reaction are written in terms of their symbols or molecular formulae on the left-hand side of the equation.
- A plus (+) sign is added between the formulae of the reactants.
- The products of reaction are written in terms of their symbols or molecular formulae on the right-hand side of the equation.
- A plus (+) sign is added between the formulae of the products.
- In between the reactants and the products an arrow sign (⟶) is inserted to show which way the reaction is occurring.
A + B ⟶ C + D
In this chemical equation, A and B are the reactants, and C and D are the products. The arrow indicates that the reaction proceeds towards the formation of C and D.
How to Balance Chemical Equations?
The first step in balancing an equation is to count the number of atoms of each element on both sides of the equation. For example, reactants X and Y2 react to form a compound XY. The word equation for this reaction would be
X + Y2 ⟶ XY
The number of atoms of elements X and Y in the above-mentioned equation is shown below.
| Element | Number of atoms in LHS | Number of atoms in RHS |
| X | 1 | 1 |
| Y | 2 | 1 |
To balance Y on both sides, multiply RHS by 2, i.e.,
X + Y2 ⟶ 2XY
Now, the number of atoms of Y is balanced but not the number of atoms of X. Therefore, multiply X on the LHS by 2. Thus, the equation becomes
2X + Y2 ⟶ 2XY
This is a balanced equation as the number of atoms of X and Y on both sides is equal.
Keeping these steps in mind, let us now write the chemical equation for the formation of magnesium oxide.
Step 1: Magnesium burns in oxygen to give magnesium oxide. Here, the reactants are magnesium and oxygen. The product is magnesium oxide.
Step 2: Thus, the word equation is
Magnesium + Oxygen ⟶ Magnesium oxide
Step 3: Replacing the names with symbols and formulae, we get the chemical equation as
Mg + O2 ⟶ MgO
Step 4: The number of atoms of the elements are
| Element | Number of atoms in LHS | Number of atoms in RHS |
| Magnesium | 1 | 1 |
| Oxygen | 2 | 1 |
To balance oxygen on both sides, multiply RHS by 2, i.e.,
Mg + O2 ⟶ 2MgO
Now, the number of oxygen atoms is balanced but the number of magnesium atoms is not. Therefore, multiply magnesium on the LHS by 2. Thus, the equation becomes
2Mg + O2 ⟶ 2MgO
This is the balanced chemical equation.
Constructing Balanced Chemical Equations
Aim: To construct balanced chemical equations.
Materials: Copper(II) carbonate powder, limewater, concentrated hydrochloric acid, concentrated
ammonia solution, lead(II) nitrate solution and potassium iodide solution.
Apparatus: Test tubes, stoppers, rubber bung with delivery tube, test tube holder, Bunsen burner and glass tube.
Procedure:
A. Heating of copper(II) carbonate
- Half a spatula of copper(II) carbonate powder is placed into a test tube.
- The apparatus is set up as shown in Figure.
Figure: Heating of copper(II) carbonate
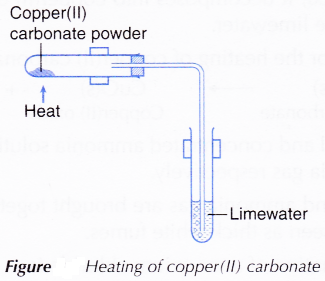
- Copper(II) carbonate is heated and the gas produced is passed through limewater.
- The changes to copper(II) carbonate and limewater are observed.
- When the reaction is completed, the delivery tube is withdrawn from the limewater and the Bunsen burner is removed.
B. Formation of ammonium chloride
- Using a glass tube, three or four drops of concentrated hydrochloric acid are dropped in a test tube. The test tube is stoppered and left aside for a few minutes.
- Using a clean glass tube, step 1 is repeated using concentrated ammonia solution.
- Both stoppers are removed and the mouths of the test tubes are brought together as shown in Figure.
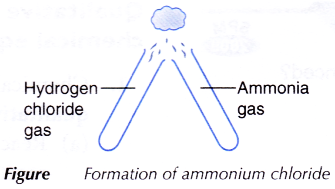
- All observations are recorded.
C. Precipitation of lead(II) iodide
- 2 cm3 of potassium iodide solution is added to 2 cm3 of lead(II) nitrate solution as shown in Figure.
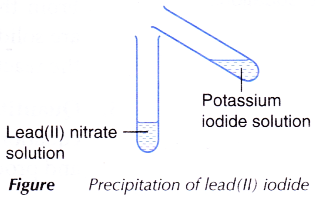
- The mixture is shaken and any change is observed.
Observations:
| Section | Observation | Inference |
| A | Copper(II) carbonate changes colour from green to black. Limewater turns milky. | Copper(II) carbonate decomposes into copper(II) oxide, which is black in colour. Carbon dioxide is released. |
| B | Thick white fumes are produced at the mouth of the test tubes. | The white fumes are solid ammonium chloride. |
| C | A yellow precipitate is produced. | The yellow precipitate is lead(II) iodide. |
Discussion:
- When copper(II) carbonate is heated, it decomposes into copper(II) oxide and carbon dioxide. The presence of carbon dioxide is detected by the limewater.
- Therefore, the balanced equation for the heating of copper(II) carbonate is
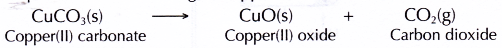
- The concentrated hydrochloric acid and concentrated ammonia solution are left for a few minutes to produce hydrogen chloride gas and ammonia gas respectively.
- When the hydrogen chloride gas and ammonia gas are brought together, they react to form fine white solids of ammonium chloride. These are seen as thick white fumes.
- The balanced equation for the formation of ammonium chloride is
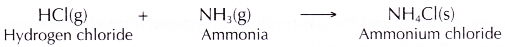
- When the colourless lead(II) nitrate solution is added to the colourless potassium iodide solution, yellow precipitate of lead(II) iodide is produced. At the same time, colourless potassium nitrate solution is also produced.
- The balanced equation for the precipitation of lead(II) iodide is

Qualitative and quantitative aspects of chemical equations
- Chemical equations give us the following qualitative information.
(a) Reactants and products of a chemical reaction.
(b) Physical states of the reactants and products. - Take the following equation as an example.
2C(s) + O2(g) ⟶ 2CO(g)
From the equation, we know that the reactants are solid carbon and oxygen gas. The product of the reaction is carbon monoxide gas. - Quantitatively, the coefficients in a balanced equation tell us the exact proportions of reactants and products in a chemical reaction.
Take the following equation as an example.
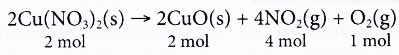
From the equation, we know that 2 moles of copper(II) nitrate decompose into 2 moles of copper(II) oxide, 4 moles of nitrogen dioxide gas and 1 mole of oxygen gas. - At the microscopic level, the coefficients in a chemical reaction tell us the number of particles 1 involved in the reaction.
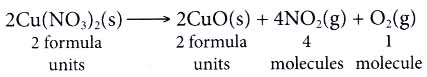
- A chemical equation serves as an important ; communicative tool for chemists.
(a) A chemical equation precisely describes a chemical reaction.
(b) Chemists use chemical equations to solve quantitatively-related problems.
Note:
- Qualititatively, hydrogen gas reacts with oxygen gas to give water.
- Qualitatively, 2 molecules (or 2 moles) of hydrogen gas react with 1 molecule (or 1 mole) of oxygen gas to give 2 molecules (or 2 moles) of water.
