What is a Chemical Change and give some Examples
Chemical change
A chemical change (also called chemical reaction) occurs when two or more chemical substances react to produce a new substance (or substances) with a different set of properties.
A chemical change is permanent and such changes are occurring all around us, even within our body. Let us look at some examples of chemical changes.
1. Reddish-brown Deposition on Iron Objects
You must have seen that if you leave an iron object such as a trowel (khurpi) or an iron chain out in the rain, a reddish-brown layer is deposited on its surface after a few days. This layer is called rust and the process is called rusting. The layer of rust falls off gradually, exposing fresh metal to further rusting. With the passage of time, the iron object becomes weak.

Let us see what actually happens.
Iron reacts with atmospheric oxygen in the presence of moisture to form a substance called iron oxide, which we call rust. The chemical equation for this reaction can be represented as:
Iron + Oxygen + Water —► Iron oxide
Rusting is a chemical change because the change is permanent. You cannot get the iron back from the oxide. Oxygen and water are two essential conditions for rusting of iron; absence of either or both of them can prevent rusting. Oiling iron objects protects their surfaces from coming in contact with moisture and air, and prevents rusting.
Activity
Aim: To show that rusting of iron requires both oxygen and water
Materials needed: Three wide-mouthed test tubes, iron nails, boiled water, tap water, anhydrous (moisture-free) calcium chloride, vegetable oil, and rubber stoppers
Method:
1. Take three test tubes and label them 1, 2, and 3 and place a clean iron nail in each of them.
2. Add a little anhydrous calcium chloride (dehydrating agent) to dry the air in test tube 1.
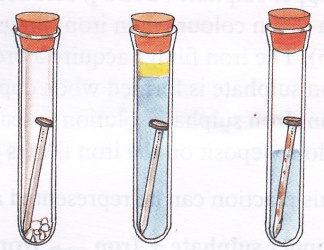 3. Pour boiled and distilled water to test tube 2 and add some vegetable oil to it to keep out the air. Boiling the water removes dissolved oxygen.
3. Pour boiled and distilled water to test tube 2 and add some vegetable oil to it to keep out the air. Boiling the water removes dissolved oxygen.
4. In test tube 3, pour a small amount of tap water.
5. Plug the mouths of all three test tubes using rubber stoppers. Keep them undisturbed for 3-4 days.
Observation: The nails in test tubes 1 and 2 have not rusted, while the one in test tube 3 has rusted.
Conclusion: As test tubes 1 and 2 did not have moisture and oxygen, respectively, there was no rusting in both these tubes. This proves that both oxygen and water are essential for rusting.
2. Browning of Vegetable and Fruit Surfaces
If you observe the cut surfaces of a brinjal, which have been exposed to the air for some time, you will find that the surfaces have turned brown. This is due to a chemical reaction between certain compounds present in brinjal and atmospheric oxygen. As a result of this reaction, a brown pigment called melanin is produced that causes browning of the surfaces.
Soaking cut vegetables and fruits in plain water can reduce the level of browning. Water would restrict the amount of oxygen that comes in contact with the surface of cut vegetables and fruits.
3. Reaction Between Vinegar and Baking Soda
Dissolve one tablespoon of baking soda powder in 100 ml of water in a beaker. Stir the mixture until all the baking soda has dissolved. Measure out 5 ml of vinegar in a separate test tube. Use a dropper to add five drops of the baking soda solution to the test tube containing vinegar. You will observe bubbles being formed due to the evolution of carbon dioxide gas.
This reaction can be represented as:
Vinegar (Acetic acid) + Baking soda (Sodium hydrogen carbonate) —► Carbon dioxide + Water + Other substances
We can test the gas that is released and find that it is carbon dioxide. Freshly prepared lime water turns milky when carbon dioxide gas is passed through it.
This reaction is also a chemical change and can be represented as:
Carbon dioxide + Lime water (Calcium hydroxide) —► Calcium carbonate + Water
4. Reaction Between Copper Sulphate Solution and Iron Filings
Copper sulphate (CuS04) solution is blue and acquires a sea-green colour when iron filings are dropped into it. The iron filings acquire a brown colour. This is because iron sulphate is formed when copper sulphate reacts with iron. Iron sulphate solution is sea-green in colour. The brown colour deposit on the iron filings is of copper.

This reaction can be represented as:
Copper sulphate + Iron —► Iron sulphate + Copper copper sulphate solution and iron filings
Activity
Aim: To study the deposition of copper from its salt solution
Materials needed: Three test tubes, copper sulphate, water, beaker, zinc metal, magnesium ribbon, and iron filings
Method:
1. Prepare an aqueous solution (solution in water) of copper sulphate in a 250 ml beaker.
2. Take three test tubes and label them from 1, 2, and 3.
3. Pour 10 ml of copper sulphate solution in each of them.
 4. Now drop a piece of zinc metal, magnesium ribbon, and few iron filings in the three test tubes, respectively.
4. Now drop a piece of zinc metal, magnesium ribbon, and few iron filings in the three test tubes, respectively.
Observation: You will observe that the blue colour of the solution will gradually disappear and ultimately become transparent in case of zinc and magnesium, whereas it will turn sea-green in case of iron. Metals in each case will be coated with a reddish-brown colour.
Conclusion: The solution turns transparent in case of zinc and magnesium due to the formation of zinc sulphate and magnesium sulphate, respectively. As iron turns into ferrous sulphate, the solution turns sea-green. The reddish coating on the metals is due to the deposition of copper metal.
