Chemical Bonding and Compound Formation
How do compounds form?
Formation of Compounds:
- Naturally occurring free elements
(a) Out of all the known elements around us, only very few elements exist naturally as free elements in the earths crust.
(b) Elements such as gold, diamond, silver, platinum, sulphur and noble gases exist naturally as free elements. - Naturally occurring compounds
(a) The other elements are usually found combined with each other in nature, forming compounds.
(b) Some examples of these naturally occurring compounds include water (H2O), carbon dioxide (CO2), natural gas (mixture of CH4, C2H6, C3H8 and C4H10), petroleum (mixture of hydrocarbons) and minerals in the earth’s crust.
(c) Minerals- Minerals in the earth’s crust usually exist in the form of oxides, sulphides, carbonates and silicates.
- Table shows the main naturally occurring compounds in some minerals.
Mineral Main compound Formula Limestone Calcium carbonate CaCO3 Bauxite Aluminium oxide Al2O3 Galena Lead(II) sulphide PbS Hematite Iron(III) oxide Fe2O3 Magnetite lron(II, III) oxide Fe3O4 Cassiterite Tin(IV) oxide SnO2 Kaolin Aluminium silicate Al2Si2O7, 2H2O Malachite Copper(II) carbonate CuCO3 Magnesite Magnesium carbonate MgCO3
- Reasons for the formation of naturally occurring compounds
(a) Elements tend to combine with each other to form compounds naturally.
(b) This is due to the formation of chemical bonds which bind the different elements together.
(c) They tend to do so because the compounds formed are more stable than the free elements. - The formation of more stable chemical bonds becomes the basis for the formation of compounds in nature.
- Physical and chemical properties of the compounds formed depend on the type of chemical bond which binds the atoms together.
- There are two main types of chemical bonds:
- Ionic bonds
- Covalent bonds
People also ask
- Chemical Bonding
- What is Covalent Bond?
- How is covalent bond is formed?
- Describe how to write a formula for a covalent compound
- What causes ions to form ionic bonds?
- Explain the formation of ionic bonds with examples
- Properties of Ionic and Covalent Compounds
- How do you write the formula for ionic compounds?
- How do you Name an Ionic Compound?
Stability of noble gases:
- Electron arrangements of noble gases
(a) Table shows the electron arrangements of noble gases (Group 18 elements).Noble gas Symbol Electron arrangement Helium He 2 Neon Ne 2.8 Argon Ar 2.8.8 Krypton Kr 2.8.18.8 Xenon Xe 2.8.18.18.8 Radon Rn 2.8.18.32.18.8 (b) All the atoms of noble gases have eight electrons in their outermost shells except helium atoms.
(c) (i) Each helium atom has only one shell filled with two electrons. This shell is completely filled, hence it is very stable.
(ii) This extremely stable electron arrangement is known as the duplet electron arrangement.
(d) (i) Except helium, the other noble gases have eight electrons in their valence shells.
(ii) This electron arrangement is known as octet electron arrangement and is very stable. - Reasons for the chemically unreactive properties
- The duplet electron arrangement of helium and the octet electron arrangement of the other noble gases are very stable electron arrangements.
- As a result, the atoms of noble gases do not accept, donate or share electrons with atoms of other elements.
- This means that the noble gas atoms will not combine with atoms of other elements to form compounds or with each other to form molecules.
- Hence, noble gases are chemically unreactive and exist as monatomic gases.
Formation of chemical bonds
- Other than noble gases which are very stable, atoms of other elements can combine among themselves or with atoms of another element through the formation of chemical bonds to achieve stable noble gas electron arrangements, that is duplet or octet electron arrangements.
- During the formation of chemical bonds, each of the combining atoms will change its electron arrangement to achieve a stable electron arrangement similar to that of a noble gas atom.
- Below is a list of the conditions for the formation of chemical bonds:
- Electrons in completely filled shells do not take part in bond formations.
- Only valence electrons are involved in bond formations.
- The combining atoms will change their electron arrangements to achieve stable noble gas electron arrangements, that is:
– achieving the duplet electron arrangement for atoms with the first shell as the outermost shell (obey the duplet rule).
– achieving the octet electron arrangement for atoms with the second/third/fourth shell as the outermost shell (obey the octet rule).
- The octet rule or duplet rule states that an atom becomes more stable during the formation of chemical bonds if its outermost shell is filled with eight electrons or two electrons for an atom with only the first shell as the outermost shell.
- During bond formation, there are two ways for the atoms of an element to achieve an octet or duplet electron arrangement. The two ways are:
(a) Transferring electrons
(b) Sharing electrons - These two ways lead to the formation of two types of chemical bonds.
(a) Ionic bond (electrovalent bond)
(b) Covalent bond - An ionic bond is usually formed when a metal combines with a non-metal.
- An ionic bond (or electrovalent bond) is the chemical bond formed through the transfer of electrons from metal atoms to non-metal atoms.
- For example, the transfer of electrons from magnesium atoms (metal atoms) to oxygen atoms (non-metal atoms) forms ionic bonds and produces the ionic compound magnesium oxide.
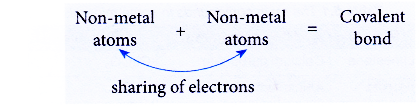
- Covalent bonds are formed when non-metal atoms combine among each other or with the atoms of another non-metal.
- A covalent bond is the chemical bond formed through the sharing of electrons between non-metal atoms.

- For example, the sharing of electrons between a hydrogen atom (non-metal atom) and a chlorine atom (non-metal atom) forms a covalent bond and produces the covalent compound hydrogen chloride.
Making Ionic Compounds Experiment
Aim: To prepare ionic compounds.
Materials: Magnesium ribbon, chlorine gas, sodium, iron filings, soda lime, sandpaper, asbestos paper and filter paper.
Apparatus: Crucible, Bunsen burner, tripod stand, pipe-clay triangle, spatula, gas jar, gas jar spoon, combustion tube, knife, forceps and stopper with delivery tube.
Safety Measures:
• Sodium is a very reactive metal. Handle sodium with care. Wear gloves and goggles when handling sodium.
• Chlorine is a poisonous gas. Do not inhale the gas.
Procedure:
A. Preparation of magnesium oxide
- A 5 cm length of magnesium ribbon is cleaned with sandpaper to remove the oxide layer on its surface.
- The magnesium ribbon is placed in a crucible as shown in Figure.
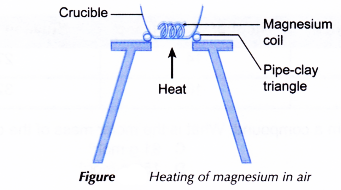
- The magnesium ribbon is heated strongly over a Bunsen burner.
- Any changes that occur are recorded.
B. Preparation of sodium chloride
- A small piece of sodium metal is cutout using a knife and forceps. The oil on its surface is wiped off by rolling it over a piece of filter paper.
- The sodium metal is placed on a gas jar spoon.
- The sodium is heated carefully in air until it starts to burn.
- The burning sodium is quickly placed into a gas jar filled with chlorine gas, a shown in Figure.
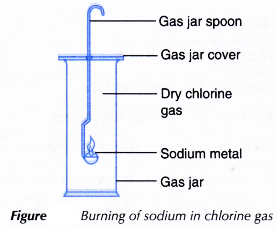
- Any changes that occur are recorded.
C. Preparation of iron(III) chloride
- One spatulaful of iron filings is placed on a piece of asbestos paper.
- The asbestos paper containing the iron fillings is placed in the combustion tube as shown in Figure.
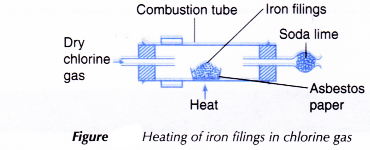
- The iron filings are heated strongly.
- Dry chlorine gas is then passed over the hot iron filings until no further changes occur.
- Any changes that occur are recorded.
Results:
| Method | Observation | Inference |
| Heating of magnesium in air | Magnesium ribbon burns rapidly with a very bright white flame. A white solid is obtained. | The white solid formed is magnesium oxide. |
| Burning of sodium in chlorine gas | Sodium burns very rapidly with a yellow flame. The greenish-yellow chlorine gas is decolourised. White fumes are liberated. On cooling to room temperature, a white solid is formed. | The white solid formed is sodium chloride. |
| Heating of iron in chlorine gas | Iron burns rapidly with a bright flame. A brown solid is formed. | The brown solid formed is iron(III) chloride. |
Discussion:
A. Preparation of magnesium oxide
- When heated, the hot magnesium ribbon reacts with oxygen in the air to produce a white solid, magnesium oxide.
2Mg(s) + O2(g) → 2MgO(s) - The metal, magnesium, combines with the non-metal, oxygen, to form an ionic compound magnesium oxide.
- Magnesium oxide consists of magnesium ions, Mg2+, and oxide ions, O2-.
B. Preparation of sodium chloride
- Sodium is a metal and chlorine is a non-metal.
- When heated, sodium reacts very rapidly with chlorine to produce a white solid, sodium chloride.
2Na(s) + Cl2(g) → 2NaCl(s) - The sodium chloride formed is an ionic compound made up of sodium ions, Na+, and chloride ions, Cl–.
C. Preparation of iron(III) chloride
- Iron is a metal and chlorine is a non-metal.
- When heated, hot iron reacts with chlorine gas to produce a brown solid, iron(III) chloride.
2Fe(s) + 3Cl2(g) → 2FeCl3(s) - The iron(III) chloride formed is an ionic compound made up of iron(III) ions, Fe3+, and chloride ions, Cl–.
Conclusion:
- When a metal combines with a non-metal, an ionic compound is formed.
- Ionic, compounds such as magnesium oxide, sodium chloride and iron(III) chloride can be prepared by direct combination of their elements.
