Chemical Bonding
We know that different elements have different atomic numbers and electronic configurations. The properties of atoms depend upon their electronic configurations. Some atoms are more reactive than others. Noble gas (He, Ne, Ar, Kr, Xe and Rn) atoms are not reactive at all; they are inert and stable. Then the question arises why noble gases do not react to form compounds, while other elements do so? This can be answered by comparing the electronic configurations of noble gases with those of other elements. Also, it is important to understand how and why atoms react to form molecules and compounds. Atoms gain electrons in their outermost shells or lose them from their outermost shells, or share electrons with other atoms in such a way that their outermost shells become filled to capacity. They can do this by reacting with other atoms. As long as the outermost shell can accommodate more electrons, i.e., it is not full, an atom tends to combine with other atoms in order to fill its outermost shell. When the outermost shell is filled to capacity, the atom becomes stable.
The atoms of all other elements (elements other than the noble gases) have in their outermost shells less than 8 electrons, i.e., their outermost shells are not filled to capacity. Therefore, the atoms of these elements combine with other atoms to achieve stable configurations like those of the noble gases. It is the tendency on the part of an atom to achieve a stable configuration (like that of the noble gases) which is responsible for its chemical reactivity.
People also ask
- Chemical Bonding and Compound Formation
- What is Covalent Bond?
- How is covalent bond is formed?
- Describe how to write a formula for a covalent compound
- What causes ions to form ionic bonds?
- Explain the formation of ionic bonds with examples
- Properties of Ionic and Covalent Compounds
- How do you write the formula for ionic compounds?
- How do you Name an Ionic Compound?
Bonds:
We know that an atom tends to attain stability by acquiring the electronic configuration of its nearest noble gas. This can be achieved in anyone of the following manners during chemical combination:
1. By the transfer of electron(s) from one atom to another
2. By the sharing of valence electrons between the two combining atoms
There must be some kind of force which binds the atoms together in a molecule. The attractive force which holds together two atoms, two molecules, two ions or a combination of these is known as a chemical bond.
The two modes of attaining the electronic configuration of the nearest noble gas give rise to two types of bonds-the electrovalent bond and the covalent bond.
The Electrovalent Bond :
In this type of bond, valence electrons are transferred from one atom to another. One atom donates its excess electrons to another atom so that both the atoms may acquire a stable noble gas configuration. The atom which loses electron becomes positively charged and is called the cation. The atom which takes up the electron lost by the first atom becomes negatively charged and is called the anion. These two oppositely charged ions are now held together by an electrostatic force of attraction. This force of attraction binding the two atoms together is known as an electrovalent or ionic bond.
Thus, the chemical bond formed between two atoms by the transfer of one or more valence electrons from one atom to the other is known as an electrovalent or ionic bond. It is also called a polar bond.
Examples: Combination of sodium (Na) and chlorine (Cl) atoms to form sodium chloride (NaCl)
The atomic number of sodium is 11. So its electronic configuration is 2, 8, 1. It has only one electron in its outermost shell. The Na atom transfers this electron and becomes positively charged sodium ion (Na+).
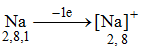 Thus, the electronic configuration of the Na+ ion is the same as that of neon which is the noble gas nearest to sodium in the periodic table.
Thus, the electronic configuration of the Na+ ion is the same as that of neon which is the noble gas nearest to sodium in the periodic table.
Let us consider the chlorine atom (Cl). The atomic number of chlorine is 17. So its electronic configuration is 2, 8, 7. It has 7 electrons in its outermost shell. It, thus, lacks 1 electron to acquire a stable noble gas configuration. So a chlorine atom takes 1 electron transferred by the sodium atom and becomes negatively charged chloride ion (Cl–).
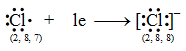 Thus, the chloride ion (Cl–) attains the configuration of the nearest noble gas, argon. [Valence electrons are shown by dots around the symbol.]
Thus, the chloride ion (Cl–) attains the configuration of the nearest noble gas, argon. [Valence electrons are shown by dots around the symbol.]
The two ions (Na+ and Cl–) being oppositely charged, are now held together by electrostatic force of attraction as Na+Cl–.
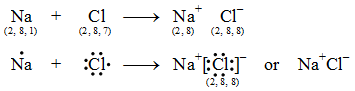 The formation of sodium chloride can be shown diagrammatically as in figure.
The formation of sodium chloride can be shown diagrammatically as in figure.
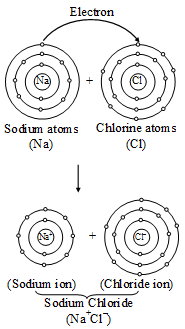 The force that holds Na+ and Cl– ions together is called an electrovalent bond. As this bond exists between ions, it is also called an ionic bond. An electrovalent bond is polar, i.e., the positive and negative charges are separated. Compounds containing such bonds are called’ electrovalent, or ionic, or polar compounds.
The force that holds Na+ and Cl– ions together is called an electrovalent bond. As this bond exists between ions, it is also called an ionic bond. An electrovalent bond is polar, i.e., the positive and negative charges are separated. Compounds containing such bonds are called’ electrovalent, or ionic, or polar compounds.
Note:
(a) In the formula of an ionic compound, the positive ion is written first,
(b) Charges on the ions of an ionic compound are usually not shown with the formula. So, sodium chloride is usually expressed as NaCl, not as Na+Cl–.
Compound | Formula | Ions involved |
Sodium chloride | NaCl | Na+ and Cl– |
Magnesium chloride | MgCl2 | Mg2+ and Cl– |
Magnesium oxide | MgO | Mg2+ and O2– |
Calcium chloride | CaCl2 | Ca2+ and Cl– |
Calcium oxide | CaO | Ca2+ and O2– |
Ammonium chloride | NH4Cl | NH4+ and Cl– |
Barium chloride | BaCl2 | Ba2+ and Cr |
Potassium nitrate | KNO3 | K+ and NO3– |
Ammonium sulphate | (NH4)2SO4 | NH4+and SO42- |
Cupric sulphate | CuSO4 | Cu2+ and SO42+ |
| Cupric chloride | CuCl2 | Cu2+ and Cl– |
Electrovalency :
When an element forms electrovalent bond, its valency is known as electrovalency.
The number of electrovalent or ionic bonds an atom can form is called its electrovalency. The electrovalency of an element is, therefore, equal to the number of electrons lost or gained by the atom to form an ion.
Elements which lose electrons show positive electrovalency and those which gain electrons show negative electrovalency. For example, in the formation of sodium chloride (Na+Cl–), the electrovalency of sodium (Na) is +1, while that of chlorine (Cl) is – l.
Elements which lose or gain one, two, three, … , etc., electrons are said to be monovalent (or univalent), divalent (or bivalent), trivalent, … , etc., respectively.
Monovalent elements : Na, CI, F
Divalent elements : Mg, Ca, Ba, O
Trivalent elements : Al, B
Characteristics of electrovalent or ionic compounds :
- Electrovalent compounds are made up of positively and negatively charged ions. For example, sodium chloride (NaCl) is made up of Na+ and Cl– ions arranged in a definite order in three dimensions to form crystals.
- Electrovalent compounds have high melting and boiling points. This is due to the presence of strong electrostatic forces of attraction between the positive and negative ions. A large amount of heat energy is required to break this force of attraction. Hence, the melting and boiling points of electrovalent compounds are high.
- Electrovalent compounds are usually soluble in water but insoluble in organic solvents such as benzene, acetone, carbon disulphide and carbon tetrachloride.
- Electrovalent compounds conduct electricity in molten state and in their aqueous solutions.
In solid electrovalent compounds the ions are held together in fixed positions and cannot move. Hence, such compounds in the solid state do not conduct electricity.
When an electrovalent compound is dissolved in water or is melted, the crystal structure breaks down. The ions now become free to move and can, therefore, conduct electricity.
That the ionic compounds in molten state or in solution become conductors of electricity.
