What are the Characteristics of Electron, Proton and Neutron
Electron
Electron was discovered by J.J. Thomson. Cathode rays consist of small, negatively charged particles called electrons. The electron is a negatively charged particle found in the atoms of all the elements. The electrons are located outside the nucleus in an atom. An electron is usually represented by the symbol e–
Characteristics of an electron
- Mass of an electron :
The mass of an electron is about of the mass of hydrogen atom. Since the mass of a hydrogen atom is 1 u. The absolute mass of an electron is, however, 9 × 10–28 gram. - Charge of an electron :
The absolute charge on an electron is 1.6 × 10–19 coulomb of negative charge. The relative charge of an electron is, – 1.
People also ask
- How would you describe the Structure of an Atom
- What was Rutherford’s Original Hypothesis
- What did Bohr Contribute to the Theory of an Atom
- Explain Bohr Bury rules for Distribution of Electrons into Different Shells
- What are the Isotopes, Isobars and Isotones of an Element
- What did Dalton Contribute to the Understanding of the Atom
- What is the Definition of Atom and Molecule
- What is Atomic Mass
- How has the Model of the Atom Changed Over the Years?
Proton
Proton was discovered by Goldstein. The proton is a positively charged particle found in the atoms of all the elements. The protons are located in the nucleus of an atom. Only hydrogen atom contains one proton in its nucleus, atoms of all other elements contain more than one proton. A proton is usually represented by the symbol P+
Characteristics of a proton
- Mass of proton : The mass of proton has been found to be equal to 1.67 × 10–27 kg. This is almost equal to that of an atom of hydrogen. Since the mass of a hydrogen atom is 1 a.m.u., therefore, the relative mass of a proton is also 1 a.m.u.
- Charge of proton : The charge of proton is equal and opposite to the charge of an electron. The value of charge on proton is 1.602 × 10–19 coulomb of positive charge.
Neutron
In 1932, Chadwick discovered the fundamental particle neutron. The neutron is a neutral particle found in the nucleus of an atom. The subatomic particle not present in a hydrogen atom is neutron. A hydrogen atom contains only one proton and one electron. A neutron is represented by the symbol n.
Characteristics of a Neutron
- Mass of a neutron : The mass of neutron is equal to the mass of a proton. The relative mass of a neutron is 1 u. The absolute mass of a neutron is 1.6 × 10–27 kg.
- Charge of an neutron : Neutron has no charge. It is electrically neutral.
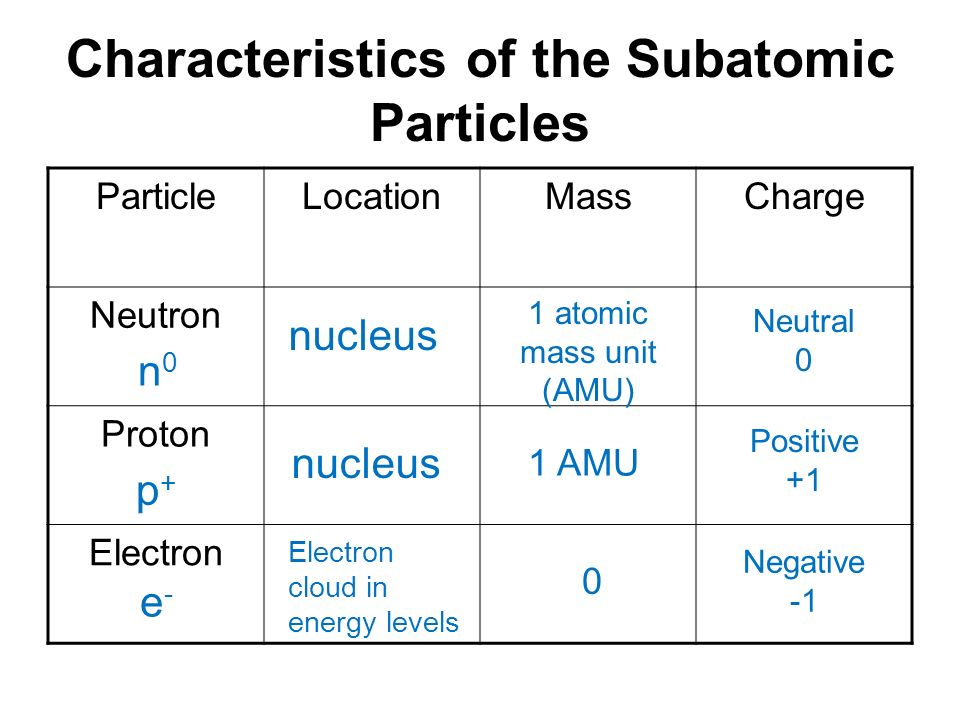
Comparision between Proton, Neutron and Electron:
S.no | Electron | Proton | Neutron |
(i) Symbol | e/e– | p/p+ | n |
(ii) Nature | Negatively charge | Positively charge | Neutral |
(iii) Relative | 1/1840 of a H atom | equal to H atom | equal to H atom |
(iv) Actual | 9.1 × 10–28 g | 1.67 × 10–24 g | 1.67 × 10–24 g |
(v) Charge | (– 1) (1.602 ×10–19 C) | (+ 1) (1.602 × 10–19 C) | No charge
|
