CBSE Sample Papers for Class 12 Chemistry Paper 5 are part of CBSE Sample Papers for Class 12 Chemistry. Here we have given CBSE Sample Papers for Class 12 Chemistry Paper 5.
CBSE Sample Papers for Class 12 Chemistry Paper 5
| Board | CBSE |
| Class | XII |
| Subject | Chemistry |
| Sample Paper Set | Paper 5 |
| Category | CBSE Sample Papers |
Students who are going to appear for CBSE Class 12 Examinations are advised to practice the CBSE sample papers given here which is designed as per the latest Syllabus and marking scheme, as prescribed by the CBSE, is given here. Paper 5 of Solved CBSE Sample Paper for Class 12 Chemistry is given below with free PDF download solutions.
Time Allowed : 3 Hours
Max. Marks : 70
General Instructions
- All questions are compulsory.
- Question number 1 to 5 are very short answer questions and carry 1 mark each.
- Question number 6 to 10 are short answer questions and carry 2 marks each.
- Question number 11 to 22 are also short answer questions and carry 3 marks each.
- Question number 23 is a value based question and carry 4 marks.
- Question number 24 to 26 are long answer questions and carry 5 marks each.
- Use log table, if necessary. Use of calculators is not allowed.
Questions
Question 1.
What type of adsorption is shown by graph showing variation of the extent of adsorption of H2 gas on charcoal with different temperature?
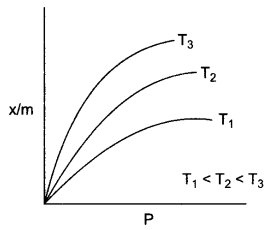
Question 2.
Draw structure of N,2-dimethylbutanamide.
Question 3.
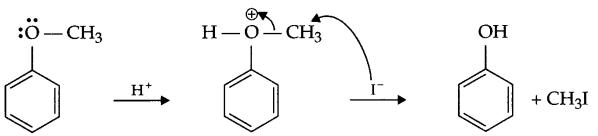
Write mechanism of the following:
Ph-O-CH3 \(\underrightarrow { HI } \)
Question 4.
Write IUPAC name of the following complex:
K[Cr(H2O)2(C2O4)2] . 3H2O
Question 5.
Define activation energy for a chemical reaction.
Question 6.
(i) What are ambident nucleophiles?
(ii) Write the product formed in the following:
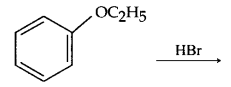
Question 7.
Draw structure of the following compounds:
(i) XeOF4
(ii) H2S2O8
Question 8.
Give reason:
- Transition metals have high enthalpy of atomisation.
- Transition metals form interstitial compounds.
Question 9.
Based on solute-solvent interactions, arrange the following in order of increasing solubility in M-octane with explanation.
Cyclohexane, KCN, CH3OH, CH3CN.
Question 10.
- p-type semiconductor
- F-centre
OR
Define osmotic pressure and why it is a colligative property?
Question 11.
Define the following:
- Glycosidic linkage
- Oligosaccharides
- Essential amino acids.
Question 12.
In elements from atomic no. 57 to 71 which element shows
- Maximum no. of oxidation state
- +2 oxidation state only (other than the common oxidation state of +3).
- Non lanthanoid nature.
Question 13.
How do you convert the following:
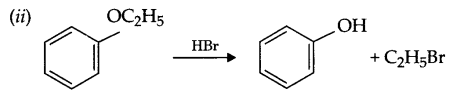
(i) Phenol to anisole
(ii) Ethanol to 2-propanol
(iii) Aniline to chlorobenzene.
OR
(i) Write mechanism of the following:
2CH3CH2OH \(\underrightarrow { { H }^{ + } }\) CH3CH2OCH2CH3
(ii) Write the reaction involved in the acetylation of salicylic acid.
Question 14.
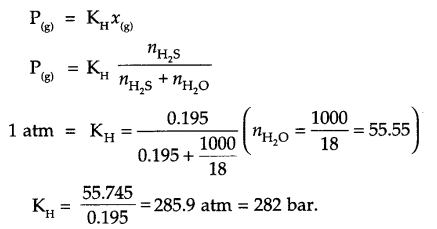
H2S a toxic gas is used for qualitative analysis. If the solubility of H2S in water at STP is
0. 195 m. Calculate Henry’s law constant.
Question 15.
Define the following terms:
- Electrophoresis
- Adsorption
- Shape selective catalysis.
Question 16.
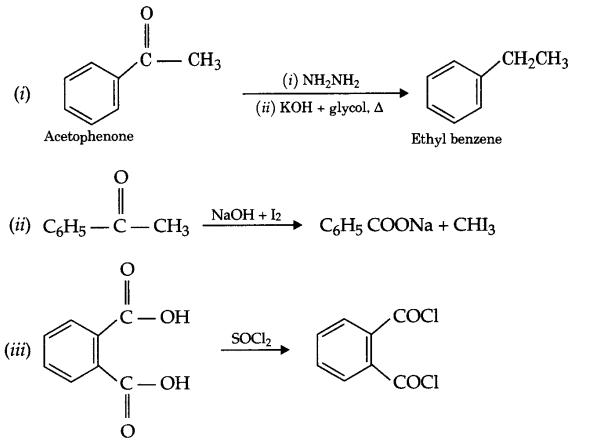
Predict the products of the following reactions:
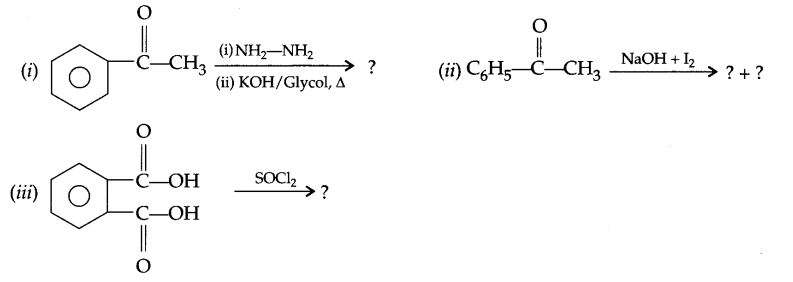
Question 17.
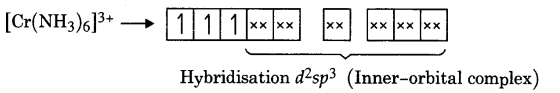
Write down the hybridisation and magnetic character of the following complexes.
(i) [Cu(CN)4]2-
(ii) [Cr(NH3)6]3+
Question 18.
Niobium crystallises in body centred cubic structure. If its density is 8.55 g/cm3. Calculate atomic radius of niobium using its atomic mass 93 u.
Question 19.
The activation energy for the reaction
2HI(g) → H2(g) + I2(g)
is 209.5 kJ mol-1 at 581 K. Calculate the fraction of molecules of reaction having energy equal to or greater than activation energy?
Question 20.
- Although thermodynamically feasible, in practice, magnesium metal is not used for the reduction of alumina in the metallurgy of aluminium. Why?
- Write down the reactions taking place in different zones in the blast furnace during the extraction of iron.
Question 21.
- What is biodegradable polymer? Give an example of biodegradable aliphatic polyester.
- Explain the difference between Buna- N and Buna-S.
Question 22.
- Write the mechanism of the following reaction:
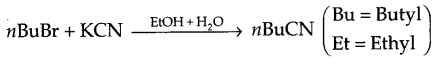
- The dipole moment of chlorobenzene is lower than that of cyclohexyl chloride.
Question 23.
Raman’s younger brother was suffering from cold, cough and fever, his father did not take him to the doctor and wanted to give medication on his own. Raman tells his father that he should not give medicine to his brother on his own but should take him to a doctor. Answer following question as per passage.
- Mention the values displayed by Ra man.
- Why should not medicines be taken without consulting doctors?
- While antacids and antiallergic drugs interfere with the action of histamine, why do thee not interfere with the function of each other?
Question 24.
(i) A voltaic cell is set up at 25°C with the following half cells.
Al | Al3+ (0.0010 M) and Ni2+ (0.50 M) | Ni
Write the equation for the cell reaction that occurs when the cell generates an electric current and determine the cell potential.
![]()
(ii) What is a fuel cell? Write reaction at anode and cathode for H2-O2 fuel cell.
OR
(i) The electric resistance of a column of 0.05 mol L-1 NaOH solution of diameter 1 cm and length 50 cm is 5.55 × 103 Ω . Calculate its resistivity, conductivity and molar conductivity.
(ii) What is a primary cell? Write cell reaction of dry cell during its discharging.
Question 25.
- Arrange the following according to given instruction.
- NO2, NO2, NO2 (Increasing bond angle)
- HF, NH3, H2O (Increasing strength of hydrogen bonding)
- CO2, SO2, NO2 (Increasing acidic strength)
- Why does nitrogen show less catenation property than phosphorus.
- Interhalogen compounds are more reactive than the halogen itself. Give reason.
OR
(a) Complete the following chemical reactions
- PH3 + HgCl2 →
- BrO–3 + F2 + 2OH– →
(b)
- Sugar gets charred on addition of concentrated sulphuric acid. Give reason with suitable chemical reaction.
- Why do noble gases have comparatively large atomic sizes?
- Why are halogens coloured?
Question 26.
(a) Explain following name reactions with a suitable chemical reaction:
- Hoffmann’s bromamide reaction,
- Carbylamine reaction
(b) Distinguish following pairs of compound with a suitable chemical test:
- Aniline and cyclohexylamine
- ropan-l-amine and propan-2-amine
- N, N-Dimethylethanamine and N-methyl ethanamine
OR
(a) Account for the following
- Primary amines have higher boiling point than tertiary amine.
- The presence of a base is needed in the ammonolysis of alkylhalide.
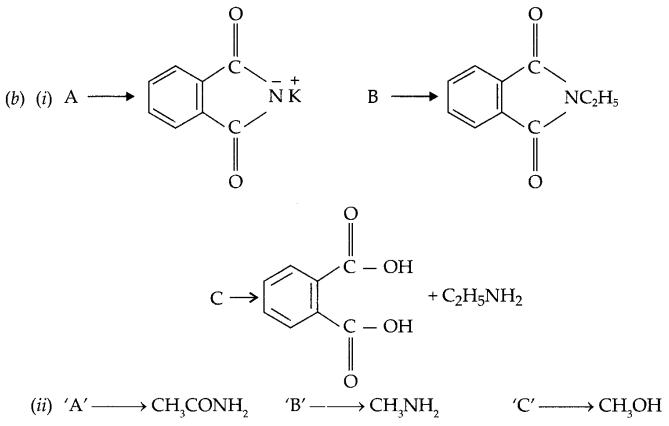
(b) Complete the following by identifying ‘A’, ‘B’ and ‘C’
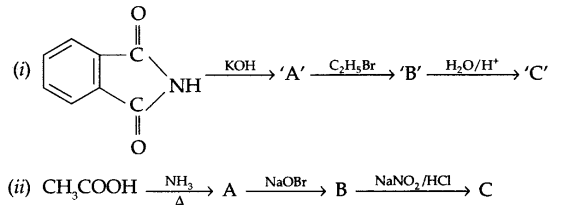
Answers
Answer 1.
Chemisorption
Answer 2.
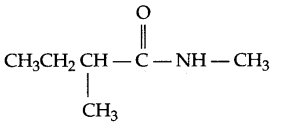
Answer 3.
Answer 4.
Potassium diaquadioxalatochromate(III) trihydrate.
Answer 5.
The minimum energy required by the reactant to get converted into product or cross over energy barrier) is known as activation energy.
Answer 6.
(i) Ambident nucleophiles: The nucleophile having two nucleophilic centres is known as ambident nucleophiles.
Answer 7.
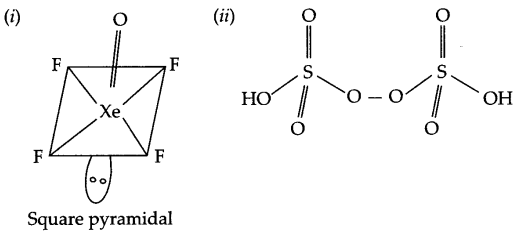
Answer 8.
- This is because of strong metallic bonds in transition metals due to presence of large number of unpaired electrons.
- The smaller atoms like H, B, C etc. can tightly fixed in interstitial space of transition metal lattice as well as they form strong metal-metal bond.
Answer 9.
KCN < CH3CN < CH3OH < cyclohexane. This trend is based on the fact that n-octane is a non-polar solvent and hence solubility of given compounds will increase with increase in covalent character.
Answer 10.
- p-type semiconductor: When silicon is doped with group 13 element like B, Ga, Al etc. then hole is created in silicon lattice which is responsible for electrical conductivity and known as p-type semiconductor.
- F-Centre: These are the anionic sites occupied by impaired electrons in the crystal lattice. F-centres are responsible for development of colour in complexes.
OR
Osmotic pressure: It is the pressure applied on higher concentration side of semipermeable membrane so that there is no net flow of solvent in either side of membrane.
Since it depends on number of solute particles in the solution so it is a colligative property.
π = CRT where, C = concentration.
Answer 11.
- Glycosidic linkage: The linkage between two monosaccharide units in a carbohydrate through oxygen atom is called glycosidic linkage. In other words, ether linkage present between two monosaccharides is known as glycosidic linkage.
- Oligosaccharides: These are the carbohydrates which on hydrolysis give two to ten units of monosaccharides e.g. sucrose, maltose, etc.
- Essential amino acids: α-Amino acid which are required for growth and proper functioning of body but are not synthesised by the human body and so must be taken in diet are known as essential amino acids.
Answer 12.
- Cerium (Ce)
- Europium and Ytterbium
- Lanthanum
Answer 13.
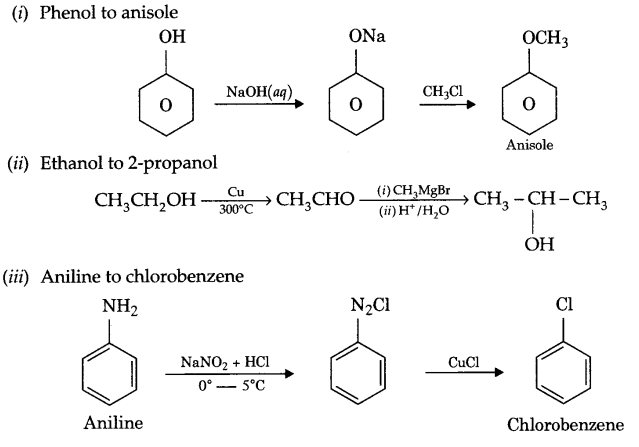
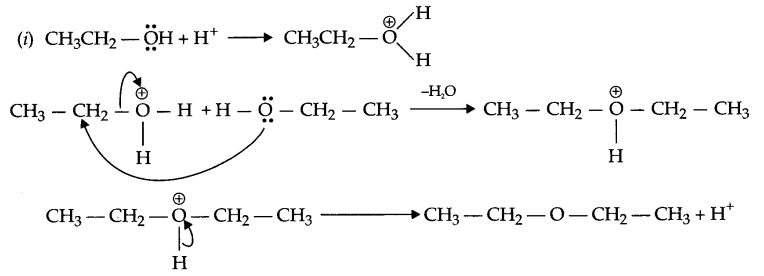
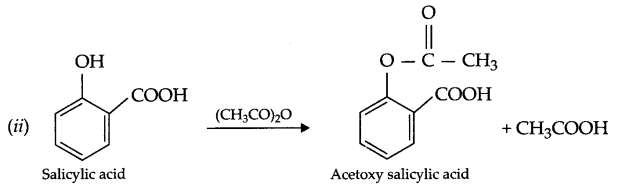
Answer 14.
Since from Henry law
Answer 15.
- The movement of colloidal particles towards oppositely charged electrode in an electric field is called electrophoresis.
- Adsorption: The phenomenon of attracting and retaining the molecules of a substance on the surface of a liquid or solid leading to a higher concentration on the surface in comparison to the inner bulk is called adsorption.
- Shape-selective catalysis: The catalysis which depends upon the structure (size) of the catalyst and molecular size of a reactant and product molecule is called shape selective catalysis e.g. zeolite.
Answer 16.
Answer 17.
(i) The electronic arrangement in [Cu(CN)4]2- according to valence bond theory:
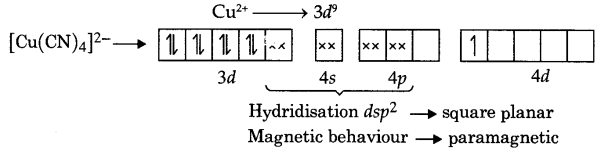
[electron present in 4d orbital is jumped from 3d orbital to provide vacant orbital in 3d orbital]
(ii) Arrangement of electrons in [Cr3(NH3)6]3+, Cr3+ is in 3d3 arrangement.
Answer 18.
Z = 2 (for bcc arrangement)
d = 8.55 g/cm3 rCa = ?
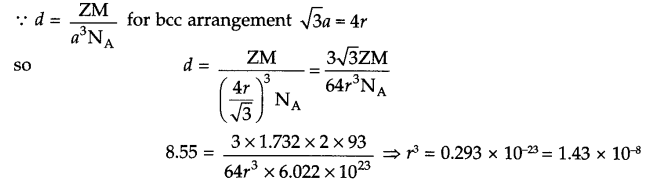
Answer 19.
The factor e-Ea/RT corresponds to the fraction of molecules possessing kinetic energy greater than or equal to Ea.
x (fraction of molecules possessing kinetic energy ≥ E ) = e-Ea/RT
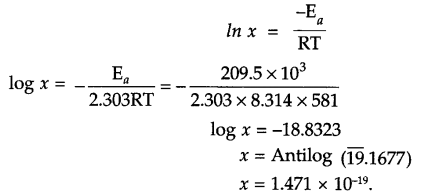
Answer 20.
- Temperatures below the point of intersection of Al2O3 and MgO curves, magnesium can reduce alumina. But magnesium (Mg) is much costlier than aluminium and hence process is uneconomical. Secondly the temperature i.e. 1350°C, Al2O3 is solid hence only partial oxidation is possible.
- Reactions in different temperature zone of blast furnace:
At 500 – 800 K
- 2Fe2O3 + CO → 2Fe2O3 + CO2
- Fe3O4 + 4CO → 3Fe + 4CO2
- Fe2O3 + CO → 2FeO + CO2
At 900 K – 1500K
- C + CO2 → 2CO
- FeO + CO → Fe + CO2
- C + O2 → CO2
Above 1570 K – coke acts as reducing agent and CaCO3 acts as flux
- FeO + C → Fe + CO
- CaCO3 → CaO + CO2
- CaO + SiO2 → CaSiO3 (Slag)
Answer 21.
- Biodegradable polymers are those which can be decomposed by microorganisms. Example – PHBV (Poly-β-hydroxybutyrate-co-β-hydroxy valerate).
- Buna-S contains monomers butadiene and styrene whereas Buna-N consists of butadiene and vinyl cyanide.
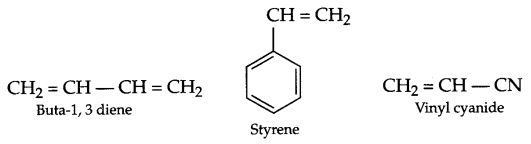
Also, Buna-S is used for automobile tyres whereas Buna-N is used for storing oils and solvents.
Question 22.
- It is substitution nucleophilic bimolecular reaction SN2.
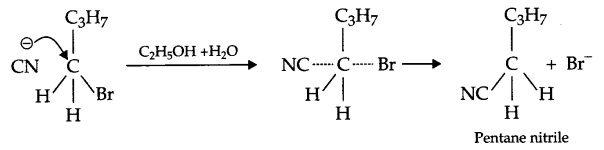
- In chlorobenzene, phenyl group is electron withdrawing and secondly due to resonance electron pair of chlorine get shifted towards benzene ring but cyclobenzyl group is neither electron withdrawing nor there is resonance hence cyclohexyl chloride has more dipole moment.
Question 23.
- Critical thinking, awareness, concern for family and knowledge of chemistry.
- Even a small dose of medicine can be proved fatal since doctor knows its proper action etc. hence medicine should be taken only on consultation with doctor.
- Target molecule of both these drugs are different hence does not interfare with one another.
Answer 24.
(i) Since reduction potential of nickel half cell is less negative it will act as reduction half cell.
So cell will be
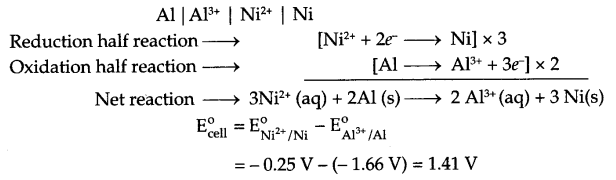
From Nernst equation at 298 k,
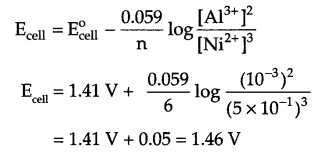
(ii) Fuel cell: The cells in which combustion energy of fuel directly get converted into electrical energy (also getting converted in any other form of energy viz. thermal energy, mechanical energy etc.) are known as fuel cell example – H2-O2 fuel cell, CH4-O2 fuel cell, NH2NH2-O2 fuel cell etc.
Reaction involving in H2-O2 fuel cell:
At anode – 2H2(g) + 4OH– (aq) → 4H2O(l) + 4e–
At cathode – O2(g) + 2H2O(l) + 4e– → 4OH– (aq)
Overall reaction – 2H2(g) + O2(g) → 2H2O (l)
OR
(i) Area of cross section of column = πr2
= 3.14 × (0.5)2 = 0.785 cm2
since \(p\frac { l }{ A } \) (Here l = 50 cm and R = 5.55 × 103 Ω )
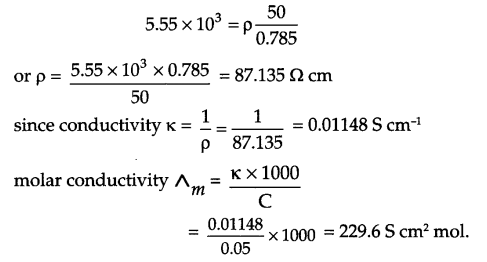
(ii) Primary cell: The cell in which reaction occurs only once to give energy and hence cannot be reversed and recharged.
Cell reaction during discharging.
Anode: Zn(s) → Zn2+ + 2e–
Cathode: (MnO2 + NH+4 + e– → MnO (OH) + NH3) × 2
Overall reaction
Zn(s) + 2 MnO2 + 2 NH+4 → Zn2+ + 2MnO(OH) + 2NH3.
Answer 25.
- NO–2 < NO2 < NO+2
- NH3 < H2O < HF
- CO2 < NO2 < SO2
- Bond enthalpy of N – N single bond is less than P – P single bond due to lone pair-lone pair repulsion in N – N hence N shows less catenation than phosphorus.
- Interhalogen compounds are more reactive reason being X – X– bonds in interhalogen compounds is weaker than X – X bond in halogens (Except F – F), and all these undergoes hydrolysis easily giving halide (from smaller halogen) and hypohalites, halites etc. from larger halogen.
OR
(a)
- 2 PH3 + 3 HgCl2 → Hg3P2 + 6 HCl
- BrO–3 + F2 + 2OH– → BrO–4 + 2F– + H2O
(b)
- Concentrated H2SO4 is strong dehydrating agent hence sugar get charred.
C12H22O11 + conc H2SO4 → 12 C + 11H2O - In case of noble gases, van der Wall radii is considered for atomic size but for other elements covalent radii is considered for this purpose hence atomic size of noble gases is comparatively larger in size.
- It is because halogens absorbs light from visible region and radiate complementary colour. (The other reason is electrons from antibonding molecular orbital are easily get excited in orbital hence halogens are coloured).
Answer 26.
(a)
- Acid amide on reaction with bromine and aqueous alkali gives primary amine having one carbon less than the amide hence it is also known as Hoffmann bromamide or Hoffmann degradation reaction.
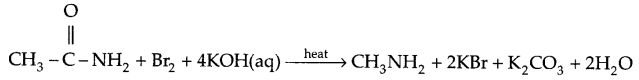
- Primary amine on reaction with CHCl2 and aqueous KOH gives isocyanide (carbyl amine) of very-very offensive smell.
RNH2 + CHCl3 + 3 KOH → RN \(\underrightarrow { = } \) C + 3KCl + 3H2O
(b) Distinguish:
1.
| Reagent | Aniline | Cyclohexylamine |
| NaNO2 + HC1, 0-5°C and than phenol | Orange or Red dye | No dye formation |
2.
Reagent | Propan-l-amine | Propan-2-amine |
| CHCl3 + KOH Heat | Offensive smell | No such smell |
| Benzene sulphonyl chloride + KOH | Solid compound form get dissolved in KOH | Solid compound formed not get dissolved in KOH |
3.
Reagent | N, N-Dimethyl ethanamine | N-methyl ethanamine |
PhSO2Cl (Benzene Sulphonyl) chloride | No reaction | Solid compound will form |
OR
(a)
- Intermolecular hydrogen bonding exist in primary amine which is not possible in tertiary amine hence primary amine has higher boiling point.
- During this reaction HCl is formed which may revert the reaction hence just to get it neutralise base like pyridine or else is used.
We hope the CBSE Sample Papers for Class 12 Chemistry Paper 5 help you. If you have any query regarding CBSE Sample Papers for Class 12 Chemistry Paper 5, drop a comment below and we will get back to you at the earliest.
