CBSE Sample Papers for Class 12 Chemistry Paper 2 are part of CBSE Sample Papers for Class 12 Chemistry. Here we have given CBSE Sample Papers for Class 12 Chemistry Paper 2.
CBSE Sample Papers for Class 12 Chemistry Paper 2
| Board | CBSE |
| Class | XII |
| Subject | Chemistry |
| Sample Paper Set | Paper 2 |
| Category | CBSE Sample Papers |
Students who are going to appear for CBSE Class 12 Examinations are advised to practice the CBSE sample papers given here which is designed as per the latest Syllabus and marking scheme as prescribed by the CBSE is given here. Paper 2 of Solved CBSE Sample Paper for Class 12 Chemistry is given below with free PDF download solutions.
Time Allowed : 3 Hours
Max. Marks : 70
General Instructions
- All questions are compulsory.
- Question number 1 to 5 are very short answer questions and carry 1 mark each.
- Question number 6 to 10 are short answer questions and carry 2 marks each.
- Question number 11 to 22 are also short answer questions and carry 3 marks each.
- Question number 23 is a value based question and carry 4 marks.
- Question number 24 to 26 are long answer questions and carry 5 marks each.
- Use log table, if necessary. Use of calculators is not allowed.
Questions
Question 1.
Write IUPAC name of
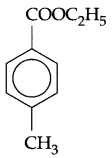
Question 2.
Which of the following compound is chiral?
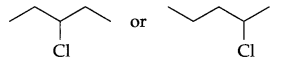
Question 3.
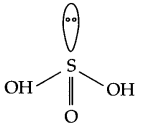
What is the basicity of H2SO3?
Question 4.
Potash alum is used to stop bleeding from cuts. Explain.
Question 5.
Find the formula of a solid if atom ‘A’ is present at the comers and atom ‘B’ occupies alternate faces of unit cell.
Question 6.
What are rare earth elements & why do these elements show variable oxidation state?
Question 7.
- Write IUPAC name for [Pt(NH3)2Cl(CH3NH2)]Cl3.
- Write formula for the compound having IUPAC name tetraaminediaqua cobalt (III) chloride.
Question 8.
Fill in the blanks:
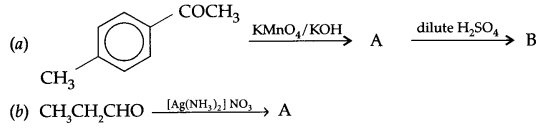
Question 9.
State Henry’s law and write its two applications.
OR
What causes positive and negative deviations from Raoult’s law? Give one example of solution for each type of deviation.
Question 10.
- Write the products of electrolysis for aqueous CuCl2 with platinum electrode.
- Define a secondary cell and give one example for it.
Question 11.
Determine the osmotic pressure of a solution in which 25 mg Al2(SO4)3 is dissolved in 400 mL of H2O at 25°C, assume 80% dissociation for Al2(SO4)3 (molar mass = 342 g/mol).
Question 12.
- Write the name of commercially most suitable metal used for getting copper from leached low grade copper ore.
- State role of graphite rod for electrometallurgy of pure alumina to get aluminium.
- Outline the principle of refining of metal by vapour phase refining.
Question 13.
An element has a body centred cubic structure having edge length of unit cell 288 pm and its density is 7.2 g/cm3. How many atoms of this element are present in its 208 g sample?
Question 14.
Account for the following:
- The enthalpy of atomisation of the transition metals is high.
- Zirconium and hafnium have almost identical radii.
- Complete the following chemical equation:
MnO–4 + SO2-4 H+→
Question 15.
(a) Draw the structure of optical isomer of [PtCl2(en)2]2+
(b) Explain the role of coordination compounds with a suitable example for the following:
- Biological system
- Metallurgy
Question 16.
Calculate emf of the following cell at 25°C
![]()
Question 17.
- What is colloidion, write its use?
- What is Latex?
- What causes “Brownian movement”?
Question 18.
Write name and structures of monomers of the following polymers:
(a) Buna-S
(b) Dacron
(c) Teflon
Question 19.
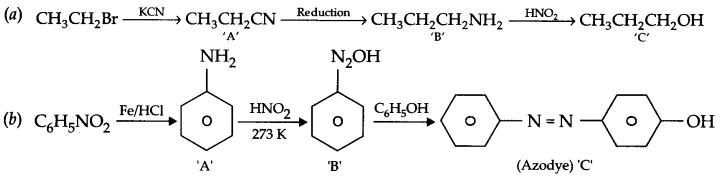
Predict the products of following reaction sequence :
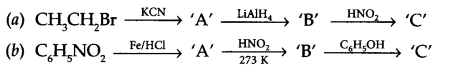
Question 20.
How will you convert the following:
(a) Phenol to 4-hydroxybenzaldehyde
(b) Chlorobenzene to p-chloroaniline
(c) Propanone to 2-methylpropan-2-ol
OR
(a) Write the mechanism of the following reaction:
CH3CH2OH ConcH2SO4→ CH2 = CH2
(b) Cyclohexanone forms cyanohydrin in good yield but 2,2,6-trimethyl cyclohexanone does not. Explain.
Question 21.
- What is glycogen? How is it different from starch?
- Write difference between nucleotide & nucleoside.
- Differentiate between globular and fibrous proteins.
Question 22.
Give one chemical test to distinguish between the following pair of compounds:
(a) Benzaldehyde and acetophenone
(b) Methanol and ethanol
(c) Benzoic acid and benzophenone
Question 23.
Chloroform is used as a solvent in the analysis of bromide and iodide ion in organic layer test. Students observed that chloroform is stored in colourless bottles, so they suggested lab incharge to store it in dark coloured bottles with 1% alcohol mixed in it.
Answer the following question according to given passage:
- Why should chloroform need to be stored in dark coloured bottle?
- Why should 1% ethanol need to be added to chloroform?
- How can you convert chloroform into tear gas?
- What are the values associated with the suggestion of students?
Question 24.
(a) Account for the following:
- BiH3 is the strongest reducing agent amongst all the trihydrides of 15th group elements.
- H2S is less acidic than H2Te.
- When HCl reacts with powdered iron, it forms ferrous chloride and not ferric chloride.
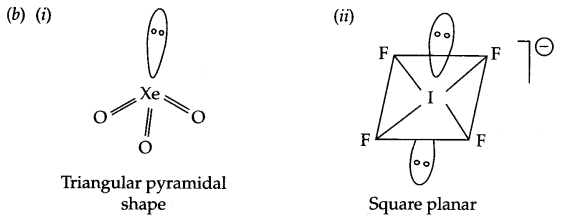
(b) Draw structures of the following:
(i) XeO3
(ii) IF–4
OR
- What inspired N Bartlett for carrying out reaction between Xe & PtF6?
- Why does N2 is used to provide inert atmosphere in some reactions.
- Halogens act as strong oxidising agent. Give reason.
- Complete the following reactions:
- NaOH (conc.)+I2 Heat→
- H2SO4 (conc.) + S(S) → ___ + ____
Question 25.
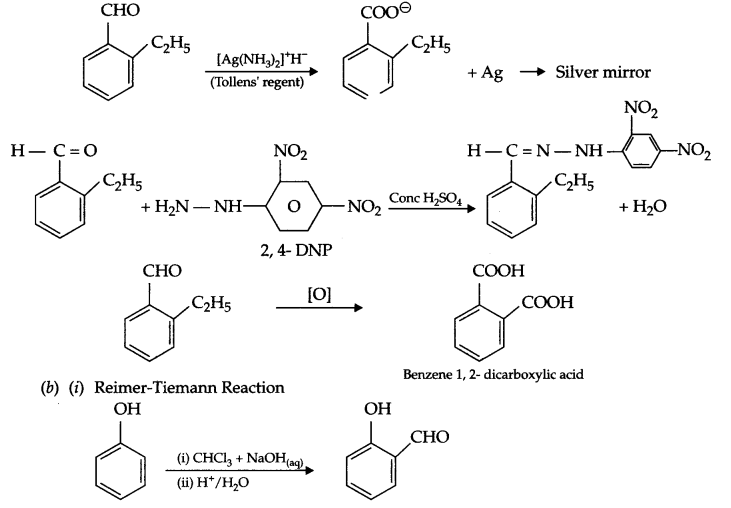
(a) An organic compound C9H10O forms 2, 4-DNP derivative, reduces Tollens’ reagent and undergoes cannizaro reaction. On vigorous oxidation, it gives 2-benzene 1,2-dicarboxylic acid. Identify the compound.
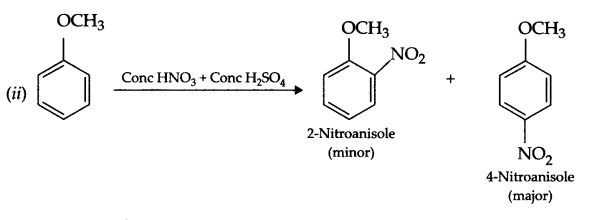
(b) Explain the following with an example
(i) Reimer – Tiemann reaction
(ii) Nitration of anisole
OR
(a) Arrange according to given information
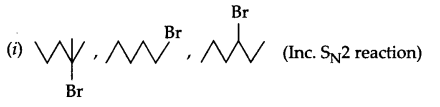
(ii) Pentan-l-ol, n-butane, pentanal, ethoxyethane (Increasing boiling point)
(iii) CH3COOH, CH2FCOOH, CH2ClCOOH, CH2FCH2COOH (Increasing acidic strength)
(b) Give the uses of Freon-12 & DDT.
Question 26.
(a) The rate constant of a reaction at 500 K and 700 K are 0.02 s-1 and 0.07 s-1 respectively.
Calculate the values of Ea and A.
(b) Define rate of a reaction and specific rate constant.
OR
(a) In a reaction between A & B the initial rate of reaction (r0) was measured for different initial concentration of A & B as given below:
| A (mol L-1) | 0.20 | 0.20 | 0.40 |
| B (mol L-1) | 0.30 | 0.10 | 0.05 |
| Rate (mol L-1 S-1) | 5.07 × 10-5 | 5.07 × 10-5 | 1.43 × 10-4 |
What is the order of the reaction with respect to A and B?
(b) Calculate half life of a first order reaction if the rate constant is
(i) 200 s-1
(ii) 4 year-1.
Answers
Answer 1.
4-methyl ethyl benzoate
Answer 2.
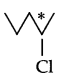
Answer 3.
Two
Answer 4.
Potash Alum (K2SO4. Al2(SO4)3.12H2O) gets dissociated into ions in aqueous solution, hence coagulates blood and stops bleeding.
Answer 5.
AB
Answer 6.
Actinoids are known as rare earth elements, reason being other than the first element all are prepared in laboratory by nuclear transmutations.
Actinoids show variable oxidation state because of comparable energy of 5f, 6d and 7s orbitals.
Answer 7.
- Diamminechloridomethanamine platinum(IV) chloride
- [CO(NH3)4(H2O)2]Cl3
Answer 8.
Answer 9.
Henry’s Law : It states that at constant temperature the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas present above the surface of the liquid or solution.
Most common form of Henry’s law :
It states that the partial pressure of the gas in vapour phase (p) is proportional to the mole fraction of the gas (x) in the solution.
p α x(g) or P = KH x(g), KH = Henry’s law constant
(Out of these two only one statement needs to be written).
Application of Henry’s law :
- Packaging of soft drink bottles. (Any two)
- Scuba divers breathing apparatus.
- Mountaineering at higher altitude.
OR
Reason for deviation from ideal behaviour : Different interactions between solvent and solute particles causes deviation from ideal behaviour.
In positive deviations, solute-solvent interactions are weaker than the interactions between solute-solute and solvent-solvent particles.
Example of positive deviation: Alcohol + H2O
In negative deviations, solute-solvent interactions are stronger than between solute-solute and solvent-solvent particles.
Example of negative deviation: HNO3 + H2O
Answer 10.
- Copper at cathode and chlorine gas (Cl2) at anode.
- A secondary cell is the one which can be recharged after use by passing current through it in the opposite direction, e.g. lead storage cell.
Answer 11.
Mass of solute (wB) = 25 mg = 25 x 10-3 g
Molar mass of soluble (mB) = 342 g/mol
Volume of solution (V) = 400 mL
Gas constant (R) = 0.082 atm L K-1 mol-1.
Temp = 25° + 273 = 298 K
Calculation for van’t Hoff factor
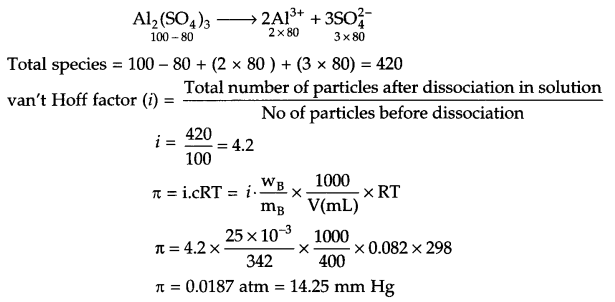
Answer 12.
- Iron scrap, due to its lower cost.
- Graphite acts as cathode as well as anode in the extraction of aluminium dining Hall Heroult process.
- Metal should form volatile complex that should be dissociated to give pure metal on further heating.
Answer 13.
For bcc arrangement, Z = 2
Density of solid (d) = 7.2 g/cm3
Mass of sample = 208 g
Edge length = 288 x 10-10 cm
No. of particles in the sample (n) = ?
d = ZMa3NA
d = Zma3n
where m = mass of sample and n = no. of particles in the sample.
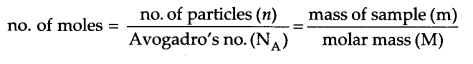
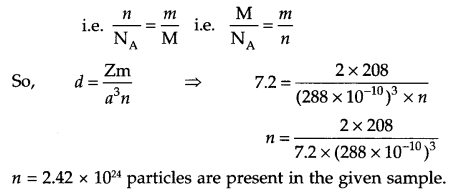
Answer 14.
- In transition metals due to small size and large number of impaired electrons metal-metal bond is strong enough that the enthalpy of atomisation is high.
- It is due to lanthanoid contraction, atomic radii of hafnium decreases to 159 pm which is quite closer to the atomic radii of zirconium, which is 160 pm.
- 2MnO–4 + 5SO2 + 2H2O H+→ 2Mn2+ + 5SO2-4 + 4H+
Answer 15.
(a) Optical isomers of [Pt(en)2Cl2]
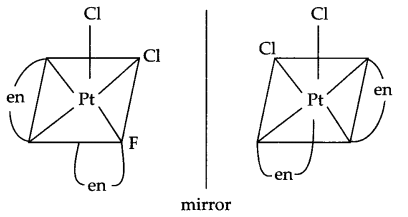
cis geometrical isomer of [Pt (en)2Cl2] show optical isomerism
(b) Applications of coordination compounds
- Biological system : Chlorophyll for photosynthesis, Haemoglobin- O2 Carrier, Vitamin B12 etc.
- Metallurgy : Extraction of Gold and Silver, Purification of Ni and Zr.
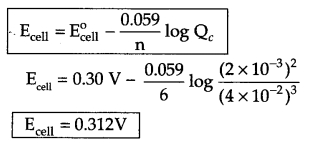
Answer 16.
![]()
Reduction half cell reaction : [Fe2+(aq) + 2e → Fe(s)]
Oxidation half cell reaction [Cr(s) → Cr3+(aq) + 3e]
Net reaction → 3Fe2+ + 2Cr(s) → 3Fe(s) + 2Cr3+
Change in number of electrons (n) = 6
According to Nernst equation at 298 K
Answer 17.
- It is a solution which is used to reduce the pore size of a filter paper so that particles of dispersed phase cannot pass through it. For this purpose 4% nitro cellulose solution is used in a mixture of alcohol and ether.
- Latex is a colloidal sol of rubber particles which is of negative charge. Rubber is obtained from it by coagulation.
- Unbalanced bombardment of particles of dispersed phase by the molecules of dispersion medium causes “Brownian movement”. This help in stabilisation of solution.
Answer 18.
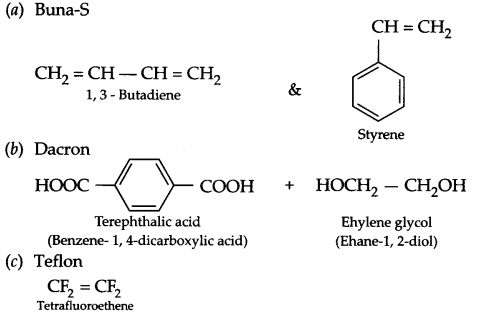
Answer 19.
Answer 20.
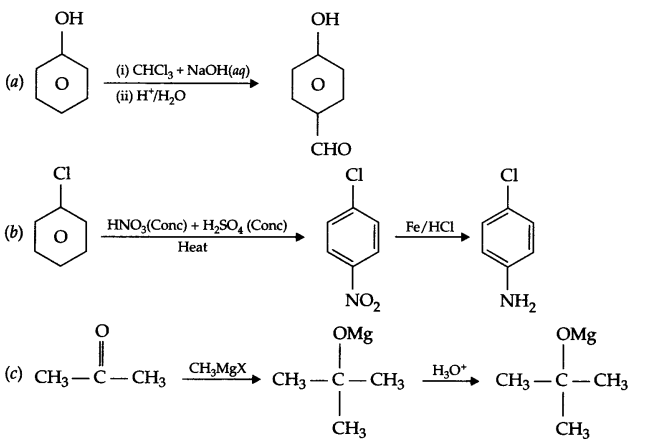
(a) Mechanism of dehydration of ethanol to form ethene:
Step-1 : Protonation of alcohol
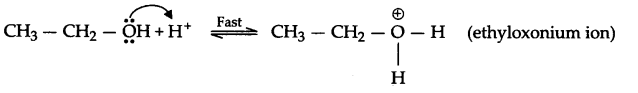
Step-2 : Formation of carbocation

Step-3 : Formation of ethene
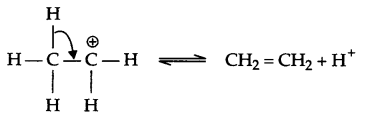
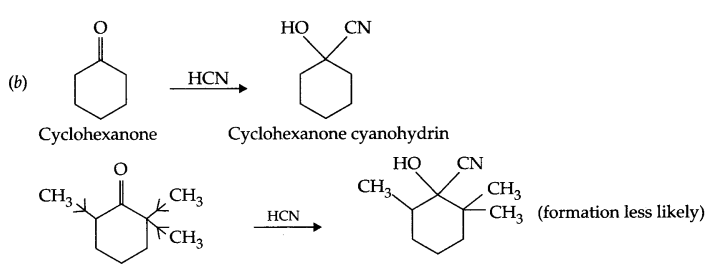
There are three methyl groups at a carbon with respect to carbonyl group, which creates steric hindrance as well as +I-effect of 3 methyl group neutralises the +ve charge on carbonyl carbon. Hence the reaction with HCN is less likely in case of 2,2,6-trimethylcyclohexanone. But there is no such hindrance in cyclohexanone so cyanohydrin forms in good yield.
Answer 21.
1. Glycogen is a form of carbohydrate stored in animal body. Its structure is similar to amylopectin of starch but somewhat more branched than amylopectin. It is a polymer of α-D-glucose.
Starch is also a polymer of α-D-glucose but it is stored in plants. It consists of two components amylose (straight chain polymer) and amylopectin (branched chain polymer).
2. Difference between nucleotide and nucleoside:
Nucleotide consists of pentose sugar, nitrogenous base and phosphate but nucleoside * consists of only pentose sugar and nitrogenous base.
3. Difference between globular and fibrous protein:
| Globular protein | Fibrous protein |
| (i) Spherical shape | (i) Thread or fibre like structure |
| (ii) Polypeptide chains get coiled together | (ii) Polypeptide chains rims parallel to one another, held together by hydrogen bonding or disulphide linkage. |
| (iii) Soluble in water (e.g. Insulin, albumin) | (iii) Insoluble in water (e.g. keratin and myosin) |
Answer 22.
(a)
Reagent | Benzaldehyde | Acetophenone |
NaOH + I2 + heat | No yellow precipitate formation | Yellow precipitate forms |
(b)
| Reagent | CH3OH | CH3CH2OH |
| I2 + NaOH + heat | No yellow precipitation | Yellow precipitate forms |
(c)
Reagent | Benzoic acid | Benzophenone |
NaHCO3 | CO2 gas evolves | No effect |
Answer 23.
- Chloroform will get oxidised to form a deadly poisonous gas, phosgene (COCl2)
- Phosgene forms ethyl carbonate with ethanol as a result there is no harmful effect.
- By treating chloroform with nitric acid.
- Dignity of individual and scientific attitude.
Answer 24.
(a)
- Bi-H bond is weakest among all 15-group hydrides.
- H-Te bond is weaker than H-S bond.
- During reaction of iron and HCl, H2 gas evolves which prevents the oxidation of FeCl2 to FeCl3.
Fe(s) + 2HCl(aq) → FeCl2(aq) + H2(g)
OR
- N. Bartlett successfuly prepared O2[PtF6]. Since ionisation energy of O2 molecule (1175 kJ mol-1) is quite closer to the ionisation energy of Xe. Hence he carried out the reaction between Xe and PtF6 which was successful and gave first noble gas compound.
- Xe + PtF6 278K→ Xe+[PtF6]–
- It is due to strong triple bond between two nitrogen atoms (N ≡ N).
- Due to weaker bond low-bond enthalpy, high negative electron gain enthalpy and highest electronegativity.
- 6NaOH + 3I2 heat→ NaIO3 + 5NaI + 3H2O
- S + 2H2SO4 → 3SO2 + 2H2O
Answer 25.
(a)
- Since given compound reacts with 2,4-DNP and reduces Tollens’ reagent, so it must be an aldehyde.
- As undergoes cannizaro reaction, means – CHO is directly attached to the benzene ring.
- On vigorous oxidation it gives benzene 1, 2-dicarboxylic acid, it means there in an alkyl group on ortho position of CHO group. This alkyl group is of 2 carbon i.e. ethyl because out of all the carbons in the compound, 6 carbon is of benzene ring and 1 of CHO, so remaining 2 carbon atoms is of this ethyl group.
Reaction involved:
OR
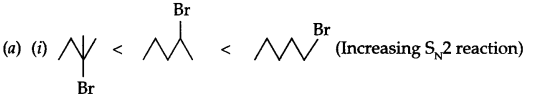
(ii) n-Butane < ethoxyethane < pentanal < pentan-l-ol (Increasing Boiling point)
(iii) CH3COOH < CH2FCH2COOH < CH2ClCOOH < CH2FCOOH (Increasing Acidic , Strength)
(b) Use of Freon-12 → Aerosol propellants, refrigeration and air conditioning.
Use of DDT → Used as insecticide particularly against mosquitoes.
Answer 26.
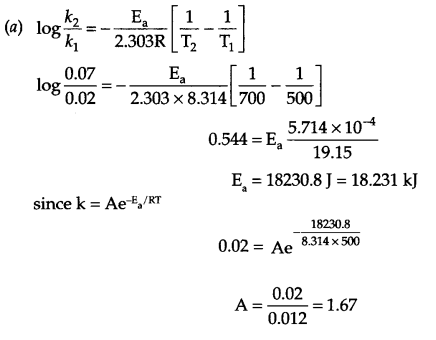
(b) Rate of a reaction : It is defined as the change in concentration of a reactant or product in unit time.
Specific rate constant : It is defined as the rate of a chemical reaction when concentration of each reactant is taken as unity.
OR
(a) Let rate law equation is rate = K[A]x [B]y where x and y are order of reaction w.r.t. A and B
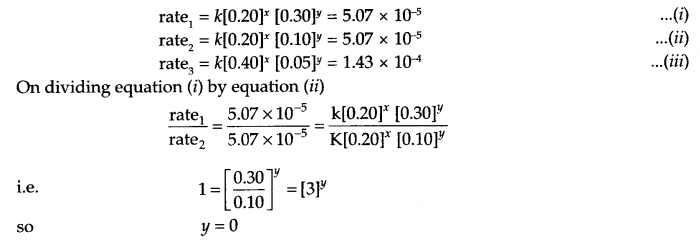
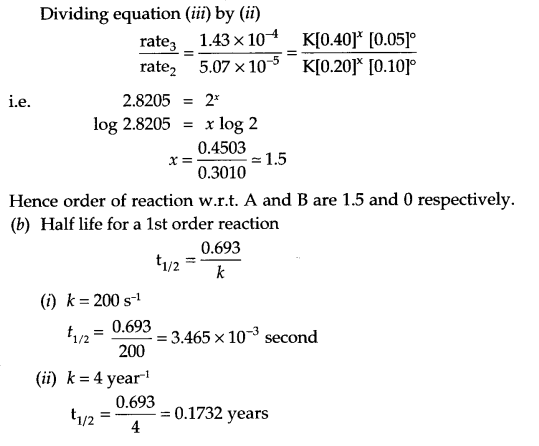
We hope the CBSE Sample Papers for Class 12 Chemistry Paper 2 help you. If you have any query regarding CBSE Sample Papers for Class 12 Chemistry Paper 2, drop a comment below and we will get back to you at the earliest.
