CBSE Class 10 Science Lab Manual – Types of Reactions
EXPERIMENT 3(a)
Aim
To perform and observe the action of water on quicklime, action of heat on ferrous sulphate crystals, reaction of iron nails kept in copper sulphate solution, reaction between sodium sulphate and barium chloride solutions and classify the reaction.
Materials Required
Calcium oxide (quicklime), distilled water, borosil beaker, test tube, glass rod, dropper, red and blue litmus paper strips.
Theory
When calcium oxide (quicklime) is dissolved in water, it forms calcium hydroxide (slaked lime). The reaction is highly exothermic, i.e. a lot of heat is produced during the reaction.
This reaction may be represented in the form of a chemical reaction as follows:

Note Calcium hydroxide is basic in nature.
Therefore, it turns moist red litmus paper blue. If we pass CO2 through clear calcium hydroxide solution (lime water), it turns milky due to the formation of a white precipitate (insoluble calcium carbonate).

In reaction (i), two compounds-quicklime and water combine to give a single product slaked lime. So, this is an example of combination reaction. Hence, it may be stated that when two or more substances react together to form a single product, is called a combination reaction.
Also, it has been observed that a large amount of heat is evolved alongwith the formation of products. Such type of reactions which are accompanied by the evolution of heat, are called exothermic reactions.
Procedure
- Take a small amount of quicklime in a borosil beaker or hard glass beaker. Slowly add water to it as shown in Fig. 1 (a).
- Stir it well with a clean glass rod as shown in Fig. 1(b).
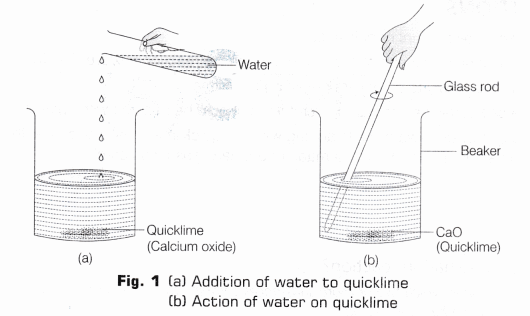
- Observe what happens. Touch the beaker carefully.
- Classify the reaction.
- Using a clean dropper, take a few drops of the solution formed in the beaker and place them on red and blue litmus paper strip (as shown in Fig. 2). Make your observation.
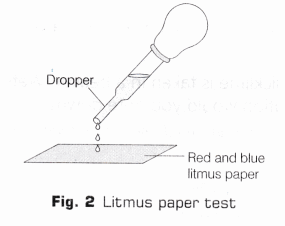
Observation
The hissing sound is produced and solution becomes hot. The heat is evolved during the reaction which raises the temperature of the reaction mixture. On putting a few drops of the solution on red and blue litmus paper, it is observed that only red litmus paper turns blue, no changes occur in blue litmus paper.
Inference
In this reaction, quicklime (CaO) and water (H2O) combine to give a single product slaked lime [Ca(OH)2 ].
CaO(s) + H2O (l) ——–> Ca(OH)2 (aq) + Heat
It is a combination reaction and calcium hydroxide [Ca(OH)2 ] is basic in nature.
As heat is evolved, the reaction is exothermic.
Result
The reaction between quicklime and water to form slaked lime is a combination reaction as well as exothermic reaction.
Precautions
- Always take small amount of quicklime.
- The reaction is highly exothermic, so do not touch the reaction mixture directly.
- Water is added slowly to a beaker containing quicklime.
- Quicklime can cause severe burns, therefore, it should be handled with spatula.
- Use good quality glassware, while adding water to quicklime. This is because, the reaction is highly exothermic and the beaker if made of ordinary glass, can crack.
Viva – Voce
Question 1.
What is combination reaction?
Answer:
A reaction in which two or more substances combine to form a single product (compound) is called a combination reaction.
Question 2.
What is calcium oxide commonly called?
Answer:
Calcium oxide (CaO) commonly known as quicklime.
Question 3.
When slaked lime is brought in contact with red litmus paper, what do you observe?
Answer:
We observe that red litmus paper turns blue. From this, we can conclude that slaked lime is basic in nature.
Question 4.
A small amount of quicklime is taken in a beaker. Water is added slowly to the beaker. What observation would you note down?
Answer:
When water is added slowly in a quicklime, hissing sound is produced and the solution becomes hot.
Question 5.
Name the reaction between calcium oxide and water.
Answer:
The reaction between calcium oxide and water is known as combination reaction.
Question 6.
Why should we not touch iron container in which CaO reacts with H2O?
Answer:
We should not touch iron container in which CaO reacts with H20 because iron container will be very hot as the reaction is exothermic.
Question 7.
Write the product formed when quicklime reacts with water.
Answer:
Quicklime reacts with water to give calcium hydroxide Ca(OH)2, ie. slaked lime.
CaO+ H2O ——–> Ca(OH)2
Question 8.
What is the effect of temperature on the solubility of calcium hydroxide?
Answer:
Solubility of calcium hydroxide or slaked lime decreases with increase in temperature.
Question 9.
Is the reaction between quicklime and water exothermic or endothermic?
Answer:
As heat energy is released during the reaction, therefore it is an exothermic reaction.
Question 10.
Name the chemical formula and chemical name of slaked lime.
Answer:
Chemical formula of slaked lime — Ca(OH)2
Chemical name of slaked lime — Calcium hydroxide
Question 11.
Most of the combination reactions are exothermic in nature. Give reason.
Answer:
Most of the combination reactions are accompanied by liberation of heat energy. This is known as exothermicity. For example, when natural gas burns in the oxygen of air, it forms carbon dioxide and water vapour. A large amount of heat is also produced.
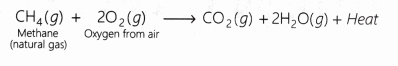
Question 12.
Give an example of a combination reaction.
Answer:
When magnesium ribbon burns in air, it forms magnesium oxide. This is an example of combination reaction.

Question 13.
How is lime water prepared?
Answer:
Mix calcium oxide with water, shake the mixture well and leave it to stand for a while. Collect the clean solution on top. This is lime water.
Question 14.
What is the suspension of slaked lime in water commonly called?
Answer:
The suspension of slaked lime in water is commonly called milk of lime.
Question 15.
Why lime water turns milky when CO2 gas is passed through a test tube containing lime water?
Answer:
Lime water turns milky due to the formation of insoluble precipitate of calcium carbonate (CaCO3).
Question 16.
Avinav takes 2 mL of clear liquid from the mixture of quicklime with water and then he breath out in it. What would he observed?
Answer:
The filtrate turns milky.
Question 17.
Out of CuO, Na2O, CaO and P2O5, in which oxide solution, lime water is formed when water is mixed?
Answer:
When water is mixed with CaO, lime water is formed
EXPERIMENT 3(b)
Materials Required
Ferrous sulphate crystals (2g), boiling tube, test tube holder, safety glass (goggle), Bunsen burner, blue and red litmus paper strips.
Theory
The ferrous sulphate crystals are actually ferrous sulphate heptahydrate (FeSO4.7H2O). They contain seven molecules of water of crystallisation. These crystals are green in colour.
Heating of Ferrous Sulphate Crystals
When the green coloured ferrous sulphate heptahydrate crystals (FeSO4.7H2O) are heated, they first lose seven molecules of water of crystallisation to form anhydrous ferrous sulphate (FeSO4 ) which is white in colour.
When this anhydrous ferrous sulphate is heated, it decomposes to give ferric oxide, sulphur dioxide and sulphur trioxide.
The reactions may be represented as:
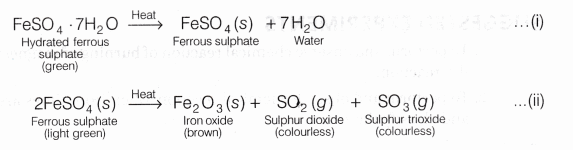
In this reaction, one substance FeSO4(s) is splitting up into three substances Fe2O3(s), SO2(g), SO3(g) because it takes place due to heat.
So, this is a decomposition reaction. It is actually a thermal decomposition reaction.
On combining Eqs. (i) and (ii), we can write the reaction as:
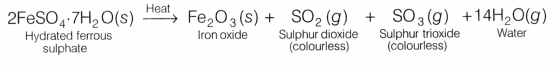
Procedure
- Take about 2g ferrous sulphate crystals in a boiling tube.
- Note the colour of ferrous sulphate crystals.
- Hold the boiling tube in a test tube holder.
- Heat the boiling tube (as shown in Fig. 1) and observe the odour of gases evolved and colour of residue formed.
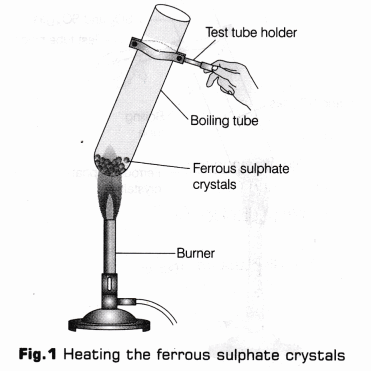
- Steamy fumes are given out which condense to form tiny droplets of a colourless liquid at the neck of boiling tube. Test the nature of these droplets with the help of blue and red litmus paper.
- Smell if any gas evolved by turning it gently towards your nose with a blow of your hand (as shown in Fig. 2).
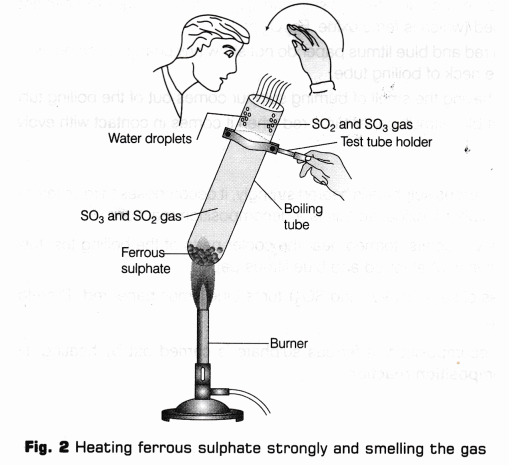
- Bring a wet blue and red litmus paper near the mouth of the boiling tube (as shown in Fig. 3) and observe the change.
- Classify the reaction.
Observation
- The green colour of ferrous sulphate crystals first changes to white and then a brown solid is formed (which is ferric oxide, Fe2O3).
- Both red and blue litmus paper do not show any change in colour with tiny droplets, formed at the neck of boiling tube.
- Gas having the smell of burning sulphur comes out of the boiling tube.
- Moist blue litmus paper turns red when it comes in contact with evolved gas.
Inference
- When ferrous sulphate is heated strongly, it decomposes to form ferric oxide, sulphur dioxide and sulphur trioxide, so this is a decomposition reaction.
- The tiny droplets, formed near the cooler parts of the boiling test tube are of water. That’s why, it is neutral to red and blue litmus paper.
- Oxides of sulphur (SO2 and SO3) turns blue litmus paper red. Therefore, gases have acidic nature.
- The decomposition of ferrous sulphate is carried out by heating, so it is called thermal decomposition reaction.
Result
Heating of ferrous sulphate crystals is a decomposition reaction and it decomposes to give ferric oxide (Fe2O3), sulphur dioxide (SO2) and sulphur trioxide (SO3).
Precautions
- Keep the mouth of the boiling tube away from yourself and your neighbour.
- Smell the gas by turning it gently towards your nose with a blow of your hand and not by bringing the mount of boiling tube under your nose.
- Wear the safety glass while performing this experiment.
- While heating FeSO4.7H2O crystals, use a hard glass test tube (boiling tube) which is perfectly dry.
- Keep the fumes of SO2 and SO3 gases away from your eyes as this may cause irritation to the eyes.
Viva – Voce
Question 1.
What is decomposition reaction?
Answer:
When a compound decomposes to give two or more simpler substances, it is a decomposition reaction. When lead nitrate Is heated, it decomposes as shown below:
![]()
Question 2.
Write the formula of ferrous sulphate crystals.
Answer:
FeSO4.7H2O
Question 3.
What is the common name of ferrous sulphate crystals?
Answer:
The common name of ferrous sulphate crystals are green vitriol
Question 4.
When FeSO4 crystals are strongly heated, it given off some gases. Name these gases.
Answer:
These gases are sulphur dioxide (SO2) and sulphur trioxide (SO3), which have suffocating smell like burning sulphur.
Question 5.
The heating of ferrous sulphate (FeSO4.7H2O) is an example of which type of reaction?
Answer:
The heating of FeSO4. 7H2O is an example of decomposition reaction.
Question 6.
What happens when crystals of ferrous sulphate are heated?
Answer:
Ferrous sulphate crystals (FeSO4.7H2O) lose water when heated and the colour of crystals changes. It then decomposes to ferric oxide (Fe2O3), sulphur dioxide (SO2) and sulphur trioxide (SO3). Fe2O3 is solid while SO2 and SO3 are gases.
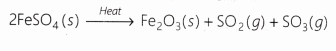
Question 7.
On heating ferrous sulphate crystals, two gases are evolved. Which gas produces fumes of sulphuric acid when it comes in contact with moist air?
Answer:
Sulphur trioxide gas produces fumes of sulphuric acid when it reacts with water vapour present in the air.
Question 8.
What residue is formed when hydrated ferrous sulphate crystals are heated?
Answer:
The residue of ferric oxide [Iron (III) oxide] Fe2O3 is formed.
Question 9.
Write the use of decomposition reaction.
Answer:
The decomposition reactions carried out by electricity, are used to extract several metals from their naturally occurring compounds like chlorides or oxides.
Question 10.
During thermal decomposition of ferrous sulphate solution, gases are produced, what is the odour of the gas?
Answer:
The gas is smell like burning sulphur.
Question 11.
What is the colour of the residue left in the test tube after thermal decomposition of ferrous sulphate?
Answer:
Brown colour residue left in the test tube.
Question 12.
Which colour of gases produces when FeSO4 crystals are strongly heated?
Answer:
A colourless gas is evolved.
Question 13.
When FeSO4 crystals are strongly heated in a test tube, what precaution should be taken and why?
Answer:
When ferrous sulphate (FeSO4) is heated in a test tube, precaution should be taken to ensure that the gas evolved, i.e. SO2 should not inhaled directly because SO2 is poisonous in nature, if one inhales this gas directly, he/she might start coughing.
EXPERIMENT 3(c)
Materials Required
Two test tubes, two iron nails, beaker, measuring cylinder, a piece of sand paper, distilled water, copper sulphate solution, thread, laboratory stand with clamp, test tube stand.
Theory
According to the reactivity series, a more reactive metal displaces a less reactive metal from its salt solution. Iron is placed above copper in the activity series. Elements placed above in this series are more reactive than those placed below them.
Thus, iron is more reactive than copper. When iron nails are placed in blue coloured copper sulphate solution, iron displaces copper from CuSO4 solution to form ferrous sulphate FeSO4, while the grey coloured iron nails gets covered by a brownish-red deposit of copper metal.
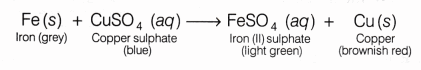
In this reaction, since the metallic iron has displaced copper ion (Cu2+ )from its salt solution, it is an example of a chemical displacement reaction. In this reaction, metallic iron is converted into ferrous iron (Fe2+) and cupric ion (Cu2+) is converted into metallic copper.

Procedure
- Take two iron nails and clean them with a sand paper.
- Take 20 ml of distilled water in a clean test tube and dissolve copper sulphate crystals in it. Label this test tube as A.
- Transfer about 10 mL of copper sulphate solution from test tube A to another clean test tube. Label this test tube as B.
- Take one iron nail tied with a thread and immerse it carefully in copper sulphate solution in test tube A for about 15 min [as shown in Fig. 1]. Keep the another iron nail separately for comparison afterwards.
- After 15 minutes, take out the iron nail from the copper sulphate solution.
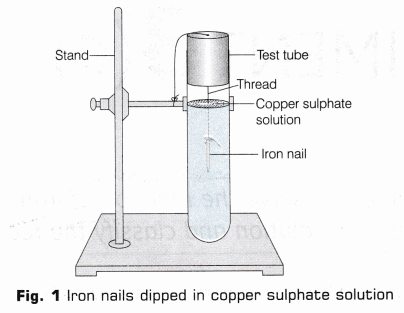
- Compare the intensity of blue colour of copper sulphate solution before and after the experiments in test tubes A and B and also compare the colour of iron nail dipped in copper sulphate solution with the one kept separately [Fig. 2], Record your observations.
Observation Table
| S. No. | Property | Before experiment | After experiment |
| 1. | Colour of copper sulphate solution | ||
| 2. | Colour of iron nail |
Result
The initial colour of copper sulphate solution in the test tube is blue and iron nails are silvery grey. After being dipped in copper sulphate solution for about 15 min, the blue colour of copper sulphate solution turns light green and the surface of iron nails get covered with brownish red coating.
It is a displacement reaction as iron displaces copper from the copper sulphate solution to form copper and ferrous sulphate.
Fe (s) + CuSO4 (aq) ———> FeSO4 (aq) + Cu (s)
Precautions
- The iron nails must be cleaned properly by using sand paper before dipping them in copper sulphate solution.
- Prepare a dilute copper sulphate solution in distilled water. If it is a concentrated solution, then it may not be possible to see the change in its colour after the experiment.
- Use good quality of boiling tube.
Viva – Voce
Question 1.
Why does the colour of copper sulphate solution change, when an iron nail is dipped in it? [NCERT]
Answer:
Iron is more reactive than copper and hence, displaces it from its salt solution.

Question 2.
How would you devise the procedure to show that Mg > Fe > Cu in reactivity series? [NCERT]
Answer:
Take ferrous sulphate solution in a beaker and add magnesium in it. Keep it undisturbed for about 5-10 min. Observe the change and draw conclusion. Now, take copper sulphate solution in another beaker and dip iron nail in it for 15 min. Observe the change. Since, magnesium can displace iron, it is more reactive than iron and again iron can displace copper, it is more reactive than copper.
Question 3.
What is the basic principle involved in this experiment? [NCERT]
Answer:
The principle involved in this experiment is that a more reactive metal can displace a less reactive metal from its salt solution.
Question 4.
Why does the following reaction takes place? [NCERT]
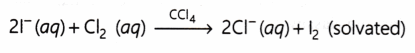
Answer:
This reaction takes place because chlorine is more reactive that iodine.
Question 5.
Iron filings were added to a solution of copper sulphate. After 10 min, it was observed that the blue colour of the solution had changed and a layer had deposited on iron filings. Which set of colours correspond to the colour of the solution and the colour of the coating respectively?
Answer:
The solution becomes light green due to the formation of ferrous sulphate and the reddish colour deposit of copper is deposited on iron filings.
Question 6.
What type of reaction is this when iron nails are dipped in copper sulphate solution?
Answer:
It is a displacement reaction, because iron is more reactive than the copper. So, iron displaces copper from CuSO4 solution.
Question 7.
Which metal gives reddish brown deposit on iron nails when dipped in copper sulphate solutions?
Answer:
Copper metal (Cu) gives reddish brown deposit on iron nails.
Question 8.
What is the correct procedure to show that iron is more reactive than copper?
Answer:
To show that iron is more reactive than copper, first prepare the copper sulphate solution and dip iron nail in it.
Question 9.
why do displacement reaction occur?
Answer:
Displacement reaction occurs due to the difference in reactivities of the elements.
Question 10.
What is reactivity series of metal?
Answer:
A series of metals in decreasing order of reactivities is called reactivity series of metals.
Question 11.
If we add silver metal in copper sulphate solution, then write the observation.
Answer:
No change is observed because silver being less reactive than copper is unable to displace copper from copper sulphate solution.
Question 12.
What is the other name given to reaction that take place between iron metal and copper sulphate solution?
Answer:
The reaction is also known as redox reaction (oxidation reduction reaction).
Because iron metal has been oxidised to Fe2+ ion, whereas Cu2+ ion has been reduced to copper metal.
Question 13.
What happens when copper is added to zinc sulphate solution?
Answer:
Copper does not displace zinc as it is less reactive than zinc.
Question 14.
In a series of reactions among metals A, B, C and D if A displaced C, B displaced A and C displaced D, then write the metals in the decreasing order of reactivity.
Answer:
B > A > C > D
EXPERIMENT 3(d)
Materials Required
Two test tubes, test tube stand, a conical flask, a glass rod, sodium sulphate solution, measuring cylinder and barium chloride solution.
Theory
Those reactions in which two ionic compounds in the solution react by exchange of their ions to form new compounds are called double displacement reactions.
The ionic compounds taken as reactants, are soluble in water. However, one of the products formed is either insoluble and separates out as a solid, called precipitate or it is a gas and other product is soluble in the solution.
When a solution of sodium sulphate is mixed with a solution of barium chloride, the following double displacement reaction takes place:
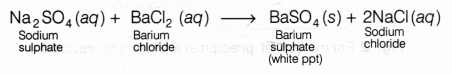
In this reaction, sulphate ions (SO4 ) from sodium sulphate are displaced by chloride ions (Cl–) and chloride ions from barium chloride are displaced by sulphate ions.
As a result, a white precipitate of barium sulphate is formed and sodium chloride remains in the solution.
Procedure
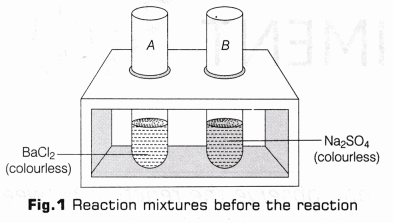
- Take two clean test tubes and label them as A and B.
- In test tube A, take the solution of barium chloride (5 ml.) and observe the colour of the solution.
- In test tube B, take the solution of sodium sulphate (5 ml) and observe the colour of the solution (as shown in Fig.1).
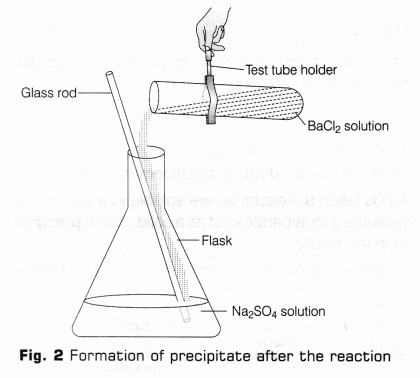
- Transfer carefully the solutions of test tubes A and B in a conical flask (as shown in Fig. 2).
- Stir the two solutions by using glass rod and leave it undisturbed for some time.
- Observe the change in colour of the solutions as per the steps given in observation table ahead.
Observation Table
| S.No. | Experiment | Observations |
| 1. | Observe the colours of both solutions in test tubes A and B before mixing them. | |
| 2. | Mix both the solutions and leave the mixture undisturbed for some time. Does anything precipitates in the test tube? if so, what is the colour of it? |
Result
Solutions of sodium sulphate and barium chloride both are colourless. On mixing the solution of sodium sulphate and barium chloride, a white precipitate is formed. This reaction is an example of a double displacement reaction, since two different salt solutions have exchanged ions to form two new salts, i.e. barium sulphate (white ppt) and sodium chloride.
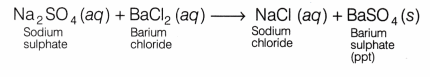
Precautions
- Test tubes, beaker and glass rod should be cleaned.
- Equal volume of sodium sulphate and barium chloride should be used.
- Add barium chloride solution very slowly to the sodium sulphate solution and stir with a glass rod.
- Do not touch or taste the chemicals.
- Do not fill more than one-third of the test tube with sodium sulphate solution.
Viva – Voce
Question 1.
What may happen on mixing Pb(NO3)2 and KCl solutions ? Predict (you may try to experimentally verify). [NCERT]
Answer:
White precipitate of lead chloride (PbCl2) will be formed on mixing Pb(NO3)2 and KCl solutions
Question 2.
Fill in the blanks
- Sodium sulphate and barium chloride are …………….. (ionic/covalent) compounds.
- As the white precipitate of barium sulphate is formed ………….. (immediately/some time) after mixing the two solutions), the reaction between ………….. (ionic/covalent) compounds is ……….. (instantaneous/slow). [NCERT]
Answer:
- Ionic
- Immediately, ionic, instantaneous.
Question 3.
What are the industrial applications of the type of reaction being studied? [NCERT]
Answer:
It is used for making compounds used in paints.
Question 4.
Why do the persons suffering from the ailment of stone formation advised not to take too much milk and tomato juice? [NCERT]
Answer:
It is because milk contains calcium and tomato has oxalate ions which form stones.
Question 5.
What type of reaction is the reaction between sodium sulphate and barium chloride?
Answer:
Double displacement reaction. The reaction can also be called precipitation reaction.
Question 6.
What do you observe when you mix aqueous barium chloride solution with aqueous sodium sulphate solution in a test tube?
Answer:
A white precipitate is formed immediately in a test tube.
Question 7.
What is the colour of Na2SO4(aq) and BaCl2(aq) ?
Answer:
Both Na2SO4(aq) and BaCl2(aq) solutions are colourless.
Question 8.
What do you mean by double displacement reaction?
Answer:
Those reactions in which two compounds exchange their ions to form insoluble compound, are called double displacement reactions.
Question 9.
Is barium sulphate (BaSO4) soluble in water or not?
Answer:
No, BaSO4 is insoluble in water.
Question 10.
Give an example (other than given in experiment) of a double displacement reaction.
Answer:
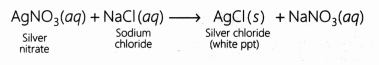
Question 11.
What is the colour of barium sulphate precipitate?
Answer:
The colour of barium sulphate precipitate is white.
Question 12.
Why is the reaction between barium chloride and sodium sulphate is double displacement reaction?
Answer:
Because in this reaction, both barium chloride and sodium sulphate exchange their ions.
Question 13.
State one commercial use of barium sulphate.
Answer:
Barium sulphate is used as a white pigment in paint industry.
Question 14.
Out of dil. HCl, dil. H2SO4 and dil. HNO3 in which acid, is barium sulphate soluble?
Answer:
Barium sulphate is not soluble in any of the above acids.
Question 15.
What would happen if dil. HCl is added to lead acetate [(CH3COO)2Pb]?
Answer:
A white precipitate of PbCl2 will be formed.
Science Lab ManualScience Practical SkillsScience LabsMath LabsMath Labs with Activity
