CBSE Class 10 Science Lab Manual – Soap Preparation
Aim
To study saponification reaction for the preparation of soap.
Materials Required
20% sodium hydroxide solution, castor oil, common salt, filter paper, distilled water, litmus paper (red and blue) strips, glass rod, borosil beakers, Bunsen burner, wire gauze and tripod stand.
Theory
- A soap is the sodium or potassium salt of fatty acids containing 15 to 18 carbon atoms, e.g. palmitic acid (C15H31COOH), stearic acid (C17H35COOH).
- Glycerides are the esters of higher fatty acids formed after combining of higher fatty acid with atrihydric alcohol, e.g. glycerol, which has three hydroxyl groups. This process is called esterification
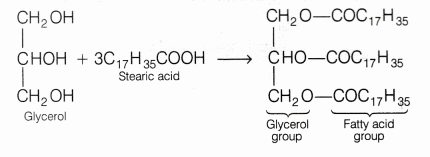
- Oil or fats (like castor oil, olive oil, palm oil) when treated with sodium hydroxide solution, these oils/fats gets converted into sodium salt of fatty acids (soap) and glycerol. This reaction is known as saponification.
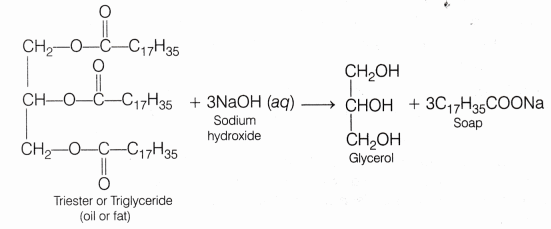
This reaction is accompanied by the evolution of heat, hence it is exothermic in nature. - Common salt is added to the mixture to make the soap come out of solution. Common salt precipitate out all the soap from the aqueous solution. Actually, when it is added to the solution, then the solubility of soap present in it decreases, due to which all the soap separates out from the solution in the form of a solid. This process is called salting out of soap.
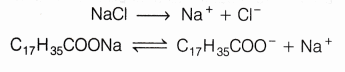
Excess of Na+ will help in the formation of soap.
Procedure
- Take 20 ml of castor oil (triglyceride) in a beaker (250 ml).
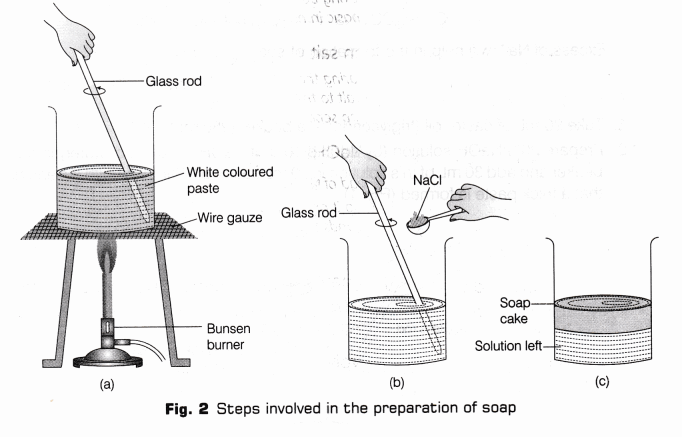
- Prepare 20% NaOH solution (by adding 10 g of NaOH in 50 ml of water) in another clean beaker and add 30 ml of this solution in 20 ml castor oil. Stir it well with a clean glass rod so that a thick paste is formed (Fig.1).
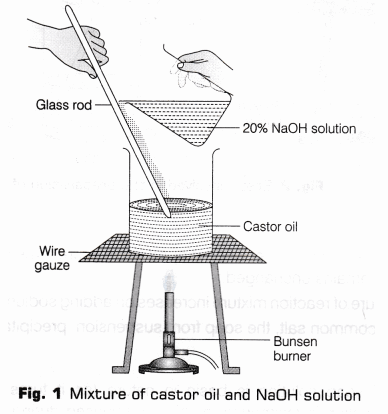
- Dip the red and blue litmus paper strip successively into this reaction mixture. Note the change in colour of litmus paper strips and record your observation.
- Touch the beaker from outside and determine whether is it hot or cold. Note the change and record your observations.
- Place the beaker on a Bunsen burner. Heat the solution with constant stirring till a thick whitish paste is formed (in suspension form) [Fig. 2(a)]. Remove the beaker from the burner and allow to cool.
Note To check whether saponification is complete or not, add few drops of the solution in a test tube containing distilled water, if the oil drops are not visible then the saponification is complete, but if oil droplets floats on the top of the surface of water that means saponification is incomplete (heating should be continued.) - Add 5 to 10 g of common salt to the above mixture and stir the mixture continuously with the
help of qlass rod till the soap begins to set. Add colour, perfume and fillers to make the soap harder [Fig. 2(b)]. - Leave the mixture for a day till it cools and becomes solid [Fig. 2(c)].
- Remove the soap cake and cut it into desired shapes and sizes.
Observation
- The colour of red litmus paper when dipped into mixture turns blue while the colour of blue litmus paper remains unchanged.
- The temperature of reaction mixture increases on adding sodium hydroxide to the castor oil.
- After adding common salt, the soap from suspension, precipitated out as a solid.
Result
The medium of soap solution is basic in nature (as it turns red litmus blue) and the saponification reaction is exothermic as heat is released during the course of the reaction. Glycerol is a by-product of this reaction.
Precautions
- Stir the soap solution carefully so that it does not spill out.
- Do not touch and taste the pellets of sodium hydroxide.
- Do not taste the castor oil.
- Handle the beaker carefully as it becomes too hot.
- Use a clean beaker and glass rod.
- Mixture of oil and NaOH solution should not be overheated.
- Continuous and thorough stirring of the reaction mixture is necessary.
Viva – Voce
Question 1.
Why does a red litmus paper changes its colour when dipped in soap solution? Explain your observation. [NCERT]
Answer:
Red litmus paper changes its colour when dipped in soap solution because soap solution is basic in nature. A soap is the salt of a strong base (sodium hydroxide) and a weak acid (carboxylic acid). So, a solution of soap in water is basic in nature, i.e. pH > 7.
Question 2.
Why is it advised to add common salt while preparing the soap? [NCERT]
Answer:
Common salt is added while preparing the soap, to precipitate out all the soap from the aqueous solution. When we add common salt to the solution, then the solubility of soap present in it decreases, due to which all the soap separated out from the solution in the form of a solid.
Question 3.
Can we use Na2CO3 instead of NaOH? Explain. [NCERT]
Answer:
No, Na2CO3 cannot be used instead of NaOH in saponification as it is a weak base, not able to break strong bond of ester and also it does not contain —OH group to participate in the reaction as —OH group containing compounds like NaOH helps in hydrolysis [decomposition of ester (triglyceride)].
Question 4.
Was heat evolved or absorbed when sodium hydroxide was added to oil? [NCERT]
Answer:
When sodium hydroxide was added to oil, then heat evolved during the reaction. It is an exothermic reaction.
Question 5.
What is the chemical reaction involved in the manufacture of soap? [NCERT]
Answer:
The chemical reaction involved is
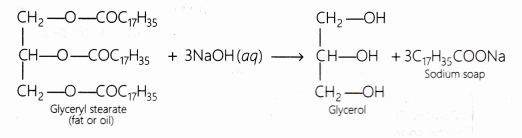
Question 6.
Can you devise a method to separate glycerine from the reaction mixture? [NCERT]
Answer:
Common salt is added to precipitate out all the soap from the aqueous solution. The solution left after the removal of soap is called lye, it contains glycerol (glycerine) which can be obtained by fractional distillation under low pressure.
Question 7.
Reena added 20% NaOH solution to 20 ml of castor oil taken in a beaker. Suddenly, the beaker becomes hot. What conclusion can you drawn from this reaction?
Answer:
The addition of NaOH solution to castor oil is an exothermic reaction as the heat is evolved during the reaction. Hence, the beaker becomes hot.
Question 8.
During commercial preparation of soap, fillers are added to it. Explain.
Answer:
For commercial preparation of soap, fillers are added as it hardens the soap and make the cutting of soap easy.
Question 9.
Name the by-product obtained in a saponification reaction.
Answer:
Glycerol (commonly known as glycerine) is obtained as a by-product in saponification reaction.
Question 10.
The reaction of castor oil with sodium hydroxide solution produces a soap molecule. Name the chemical composition of this soap molecule.
Answer:
Castor oil contains glyceryl oleate. Thus, the soap molecule prepared from the reaction of castor oil with sodium hydroxide solution is sodium oleate.
Question 11.
Give two examples of soap.
Answer:
Sodium palmitate (C15H31COONa) and sodium stearate (C17H35COONa).
Question 12.
What are esters?
Answer:
Esters are organic compounds having general formula
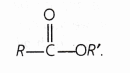
e.g. CH3—COOC2H5 (Ethyl ethanoate)
Question 13.
What is a fatty acid?
Answer:
A fatty acid is a carboxylic acid which consists of a long unbranched, saturated or unsaturated aliphatic chain.
Question 14.
Apart from sodium hydroxide, name the other base which can be used for preparing soap.
Answer:
Potassium hydroxide
Question 15.
What is the use of glycerol?
Answer:
Glycerol is used for pharmaceutical industry.
Question 16.
What are glycerides?
Answer:
Esters of higher fatty acids formed with glycerol are called glycerides.
Question 17.
Name the raw materials used in soap industry.
Answer:
Raw materials used in soap industry are:
- Vegetable oil
- Caustic soda
- Common salt
Question 18.
How the temperature of reaction mixture vary when we add sodium hydroxide solution to oil?
Answer:
The temperature will increase on adding sodium hydroxide solution to oil.
Question 19.
What will be the product formed, when groundnut oil is Heated with caustic soda solution?
Answer:
A soap is formed.
Question 20.
Why does soap solution appears cloudy?
Answer:
Soaps micelles scatter light because of their large size.
Science Lab ManualScience Practical SkillsScience LabsMath LabsMath Labs with Activity
