CBSE Class 10 Science Lab Manual – Properties of Acids and Bases
EXPERIMENT 2(a)
Aim
To study the properties of acids (dil. HCl) and bases (dil. NaOH) by their reaction with
- Litmus solution (blue/red)
- Zinc metal
- Solid sodium carbonate
Materials Required
Test tubes, test tube stand, test tube holder, cork, droppers, boiling tube, match-box, burner, flat bottom flask, thistle funnel, beaker, litmus solution/paper (red and blue), glass rod, zinc granules, freshly prepared lime water, solid sodium carbonate and dil. HCl.
Theory
Acid
An acid is a substance which furnishes H+ ions when it is dissolved in water like HCl. Acids turn blue litmus red and do not affect red litmus.
On reacting with zinc metal, HCl forms a salt, zinc chloride (ZnCl2) and hydrogen gas (H2) is liberated.
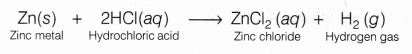
Hydrogen gas burns in air with a blue flame and produces a pop sound.
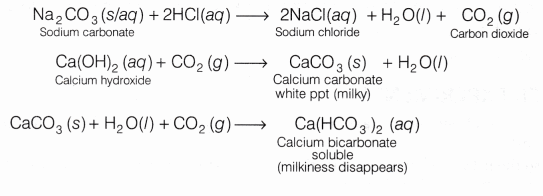
HCl reacts with sodium carbonate (aqueous/solid) to liberate carbon dioxide (CO2 ) which turns lime water milky due to the formation of calcium carbonate. When excess of CO2 is passed through the solution, the milkiness disappears.
Procedure
- Litmus Test
- Take two test tubes and mark them A and B and put them in a test tube stand. In test tube A take 5 ml of blue litmus solution and in B, take 5mL of red litmus solution (as shown in Fig.1).
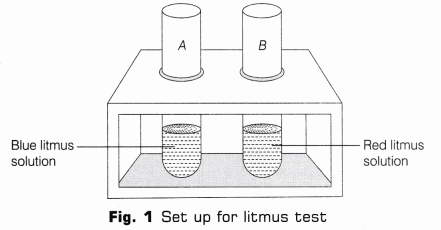
- Using a dropper, add few drops of HCl in each test tube. Stir each tube with separate glass rods B. Note, if there is any colour change occurs in the solutions.
Observation In test tube A, blue litmus turns red and nothing happens to red litmus. Hence, it can be concluded that acids (like HCl) turns blue litmus to red.
- Take two test tubes and mark them A and B and put them in a test tube stand. In test tube A take 5 ml of blue litmus solution and in B, take 5mL of red litmus solution (as shown in Fig.1).
- Reaction with Zinc (Zn) Metal
- Take few zinc granules in a clean and dry test tube.
- Add HCl to the test tube containing zinc granules, such that zinc granules are totally submerged in the acid.
- Place a cork having glass delivery tube.
- A vigorous reaction will take place after 2-3 minutes, with the evolution of a colourless, odourless gas.
- On bringing a burning match-stick near the mouth of gas tube, the gas burns with a pale blue flame producing pop sound (as shown in Fig. 2).
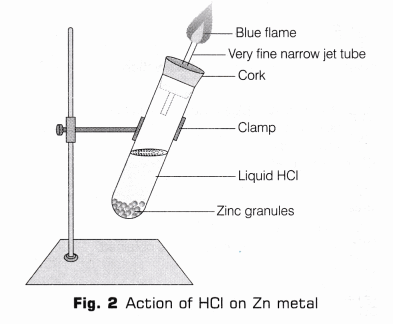
Reaction 2HCl(aq) + Zn(s) ——> ZnCl2 (aq) + H2↑
Observation Acids (like HCl) liberate hydrogen (H2) gas on reacting with active metals like zinc (Zn) which burn with a pop sound when burning splinter brought near to it.
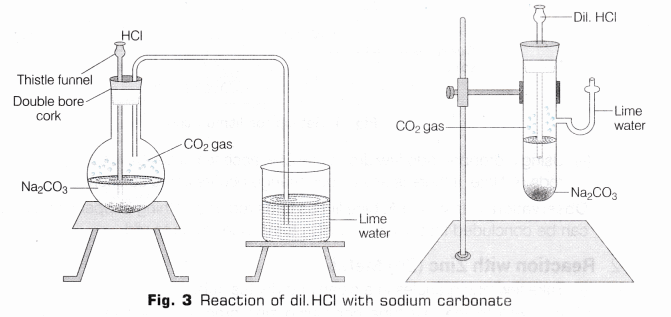
- Reaction with Solid Sodium Carbonate (Na2CO3)
- Take a small amount of solid sodium carbonate in a flat bottom flask and some distilled water in it, shake it well.
- Take a double bore cork with a thistle funnel and a delivery tube fitted in it, fit it on the open end of the flask.
- Now add HCl in the flask through thistle tube.
- Reaction taken place with the evolution of colourless and odourless gas. Then gas is passed through freshly prepared lime water with the help of delivery tube (as shown in Fig. 3).
- The lime water turns milky.
Reaction
Na2CO3(aq)+2HCl(aq) ——–> 2NaCl(aq)+ CO2 ↑ + H2O(l)
Ca(OH)2 (aq) + CO2 ↑ ——–> CaCO3(s) + H2O (l)
CaCO3 (s) + H2O(l) + CO2 (g) ——–> Ca(HCO3)2 (aq)
Observation HCl on reacting with sodium carbonate (Na2CO3) liberates carbon dioxide (CO2) gas which turns lime water milky. On passing the gas in excess of lime water, the milkiness disappears.
Observation Table
| S.No. | Experiment | Observation | Inference |
| 1. | Litmus test Add few drops of dil. HCl to test tube A containing blue litmus solution. Add few drops of dil. HCl to test tube B containing red litmus solution. | Blue litmus solution turns ………….. in colour. | HCl has……. character. HCl…….. affect red litmus solution. |
| 2. | Reaction with Zn metal
|
|
|
| 3. | Reaction with Na2CO3 Add a few drops of dil. HCI to Na2CO3. | A colourless, odourless gas is evolved which turns lime water milky. On passing the gas in excess in lime water, the milkiness disappears. | ……. gas is liberated on the reaction between dll. HCl and Na2CO3. 2HCI + Na2CO3 ——–> 2NaCl +……… + H2O |
Result/Conclusion
- Hydrochloric acid turns blue litmus solution/paper to red but it does not affect red litmus solution/paper.
- It reacts with zinc metal to liberate hydrogen gas and also forms zinc chloride as a product.
- It reacts with sodium carbonate to liberate carbon dioxide.
Hence, we conclude that hydrochloric acid is acidic in nature.
Precautions
- As HCl is corrosive in nature, it should be handled with care.
- Use small quantities of chemicals.
- Use small quantities of Zn and HCl, otherwise large amounts of H2 will be formed which may cause explosion.
- Use clean zinc metal, otherwise the reaction will occur very slowly.
- Add HCI to Na2CO3, when the apparatus is airtight.
- Observe the milkiness in the lime water soon.
- In case you allow carbon dioxide to pass for a long time through lime water, the milkiness may be removed due to the formation of soluble calcium bicarbonate as depicted in the reaction as follows:
CaCO3(s)+ CO2(g)+ H2O(l) ———> Ca(HCO3)2(aq). - Shake the solutions and reaction mixtures carefully without spilling.
- Care must be taken while performing combustion test with H2.
Viva-Voce
Question 1.
What will be the colour of a blue litmus paper on bringing it in contact with a drop of dil. hydrochloric acid? [NCERT]
Answer:
The colour of the blue litmus paper changes to red on bringing it in contact with a drop of dil. hydrochloric acid.
Question 2.
Explain, why hydrogen gas is not collected by the downward displacement of air? [NCERT]
Answer:
Hydrogen gas is not collected by the downward displacement of air because hydrogen gas is lighter than air.
Question 3.
What will happen to a lighted candle if it is brought near the mouth of a gas jar containing hydrogen gas? [NCERT]
Answer:
The candle extinguishes with a pop sound and the gas burns with a pale blue flame.
Question 4.
Which gas is produced when zinc metal reacts with hydrochloric acid? [NCERT]
Answer:
When zinc metal reacts with hydrochloric acid, hydrogen gas is produced.
Zn(s)+ 2HCl(aq) ——> ZnCl2(aq)+ H2(g)
Question 5.
Which gas is liberated when sodium carbonate reacts with hydrochloric acid? [NCERT]
Answer:
When sodium carbonate (Na2CO3) reacts with hydrochloric acid, carbon dioxide (CO2) gas is released.
Na2CO3(s)+ 2HCl(aq) ———> 2NaCl(aq)+ CO2(g)+ H2O(l)
Question 6.
Hydrogen gas is neutral to litmus paper. Explain how? [NCERT]
Answer:
Hydrogen gas is neither acidic nor basic in nature, thus it does not affect blue and red litmus paper.
Question 7.
What is the utility of the reaction between NaHCO3 and HCl in daily life situation? [NCERT]
Answer:
Sodium bicarbonate (NaHCO3) acts as an antacid and it neutralises excess of hydrochloric acid (HCl) in our stomach.
Question 8.
How can the deposits of carbonates and hydrogen carbonates on the metal surface be cleaned? [NCERT]
Answer:
The deposits of carbonates and hydrogen carbonates on the metal surface can be cleaned by dil. HCl or acetic acid.
Question 9.
Name one metal (other than zinc) which on reacting with dil. HCl gives a colourless gas that burns with a pop sound.
Answer:
Magnesium metal evolves hydrogen gas on reacting with dil. HCl. The gas burns with a pop sound.
Question 10.
When a white powder was mixed with dilute acid, a colourless and odourless gas was produced which turned lime water milky. What is this white powder?
Answer:
White powder may be carbonate or bicarbonate of metals, which evolve CO2 on reacting with dilute acid. CO2 gas turned lime water milky
Question 11.
Why does lime water turn milky when CO2 is passed through it?
Answer:
It is due to the formation of insoluble calcium carbonate.
Question 12.
If a moist blue litmus paper is brought near CO2 gas. What change would be observed on the blue litmus paper?
Answer:
Blue litmus paper turns red. In the presence of moisture, C02 changes to carbonic acid.
CO2 + H2O ——> H2CO2
It will change the colour of blue litmus to red.
Question 13.
Why do metal displace hydrogen from dilute acids?
Answer:
It is because metals are more reactive than hydrogen, therefore they can displace H2. They can supply electrons to convert H+ ions to H2 gas.
2H+ + 2e– ———> H2(g)
Question 14.
Which substance will be required to identify the gas evolved when dil. HCl reacts with solid sodium carbonate?
Answer:
Lime water will be required. CO2 is evolved in the reaction which turns lime water milky.
Question 15.
A drop of red litmus solution is poured in a metal carbonate solution. The colour of litmus solution change to blue. What is the nature of metallic carbonate solution?
Answer:
The metallic carbonate solution is alkaline in nature.
Question 16.
Out of acetic acid and hydrochloric acid, which would have a lower pH?
Answer:
HCI would have lower pH as it can easily dissociate to give more H+ ions as compare to acetic acid.
EXPERIMENT 2(b)
Materials Required
Test tubes, test tube stand, test tube holder, litmus solution/paper strips (red and blue), dil. NaOH, solid sodium carbonate, zinc granules, Bunsen burner, distilled water, match-box, thistle funnel, flat bottom flask, delivery tube, cork, glass rods and freshly prepared lime water.
Theory
Base
A base is a substance which furnishes OH– ions when it is dissolved in water. Sodium hydroxide (NaOH) is a strong base. Its pH is much higher than 7.
So, it turns red litmus into blue and do not affect the blue litmus.
On reacting with Zn metal, it forms a salt (sodium zincate) and hydrogen gas (H2) is liberated.
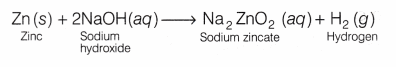
Solid Na2CO3 does not react with NaOH because both are basic in nature.

Base (NaOH) neutralizes acid (HCl) to gives salt (NaCl)
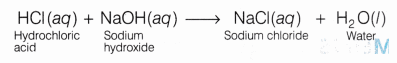
Procedure
- Litmus Test
- Take two test tubes and mark them A and B. Take 3 mLof blue litmus in test tube A and 3 mL of red litmus in test tube B and put them in a test tube stand (as shown in Fig. 1).
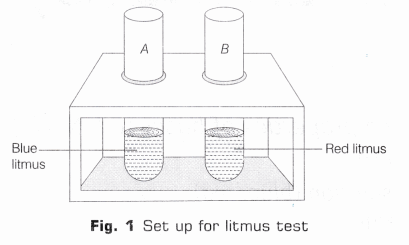
- Using a dropper, add few drops of NaOH in each. Note, if there is any colour change in the solutions in the test tubes.
Observation Red litmus in test tube B turns into blue while there is no change in blue litmus present in test tube A. Hence, it can be concluded that base like NaOH turns red litmus to blue.
- Take two test tubes and mark them A and B. Take 3 mLof blue litmus in test tube A and 3 mL of red litmus in test tube B and put them in a test tube stand (as shown in Fig. 1).
- Reaction with Zinc (Zn) Metal
- Take few zinc granules in a clean and dry test tube,
- Add NaOH to the test tube containing zinc granules, such that zinc granules are totally submerged in the acid.
- Place a cork having glass delivery tube and heat the test tube.
- A vigorous reaction will take place after 2-3 minutes with the evolution of a colourless, odourless gas.
- On bringing a burning match-stick near the mouth of gas tube, the gas burns with a pale blue flame producing pop sound (as shown in Fig. 2).
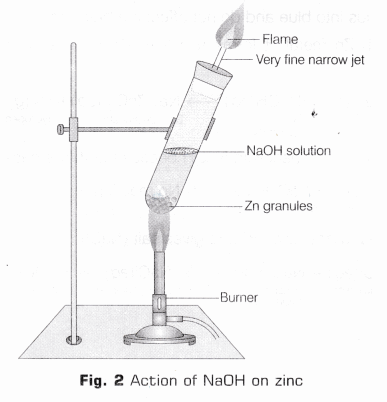
Reaction
2NaOH(aq) + Zn(s) ——> Na2ZnO2 (s) + H2↑
Observation Base like NaOH liberates H2 gas on reacting with active metals like Zn which burns with pop sound when burning splinter brought near to it.
3 Reaction with Solid Sodium Carbonate (Na2CO3)- Take a small amount of Na2CO3 in a test tube.
- Add some drops of dil. NaOH solution in the test tube containing Na2CO3.
- No reaction takes place.
Observation Table
| S. No. | Experiment | Observation | Inference |
| 1. | Litmus test
|
|
|
| 2. | Reaction with Zn metal
|
|
|
| 3. | Reaction with Na2CO3 Add a few drops of dil. NaOH to Na2CO3. | ……change is observed. | NaOH and Na2CO3 do not react. |
Result/Conclusion
- Sodium hydroxide turns red litmus solution/paper blue but it does not affect blue litmus solution/paper.
- It reacts with zinc metal and liberates hydrogen gas. During this reaction, sodium zincate is also formed.
- It does not react with sodium carbonate.
Hence, we conclude that sodium hydroxide (NaOH) is basic in nature.
Precautions
- Sodium hydroxide should be handled with care because it is highly corrosive in nature. Do not heat mixture of zinc and dilute NaOH to boiling point.
- Small quantity of chemicals should be used to perform the experiment to get best results. Test tubes and droppers should be washed well with distilled water before use.
- Do not interchange droppers while testing with acids/alkalis/indicators.
- Hands must be cleaned properly after completing the experiment.
Viva-Voce
Question 1.
What will be the colour of blue litmus paper on bringing it in contact with a drop of dil. NaOH? [NCERT]
Answer:
The colour of the blue litmus paper remains blue on bringing it in contact with a drop of dil.NaOH.
Question 2.
Which gas is produced when aluminium metal (Al) reacts with sodium hydroxide? [NCERT]
Answer:
Hydrogen gas (H2) is produced when aluminium metal (Al) reacts with sodium hydroxide.
Question 3.
What are the metals (other than Al) which react with alkalies to produce hydrogen gas? What are these metals called? [NCERT]
Answer:
Zinc. These metals are called amphoteric metals.
Question 4.
What are strong bases? Give example.
Answer:
Those bases which dissociate into its ions completely are called strong bases, e.g. NaOH, KOH etc.
Question 5.
A blue litmus paper was first dipped in dil. HCl and then in dil. NaOH solution. What colour would you observe on the litmus paper?
Answer:
Dilute HCl turns blue litmus paper red which becomes blue again in dil. NaOH. solution.
Question 6.
A teacher gave two test tubes, one containing water and the other containing sodium hydroxide solution, to the students and asked them to identify the test tube containing sodium hydroxide solution. Which substance can be used to identify the test tube?
Answer:
Red litmus paper can be used.
(Red litmus paper turns blue in the presence of sodium hydroxide solution)
Question 7.
What will be the reaction of Zn with NaOH on heating?
Answer:
Zn reacts with NaOH to form sodium zincate and hydrogen gas.
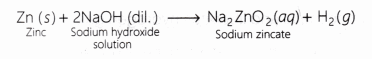
Question 8.
What will be the effect of litmus on hydrogen gas?
Answer:
Hydrogen gas is neutral towards litmus. It is neither acidic nor basic therefore, it neither changes the colour of blue litmus nor red litmus.
Question 9.
A yellow stain of turmeric turns red when rubbed with soap. What is the reason behind this change?
Answer:
The yellow stain of turmeric changes to red because soap is alkaline in nature, since turmeric (an indicator) changes to red colour in the presence of OH– ions.
Question 10.
When carbon dioxide is passed through lime water, it turns milky. Due to which insoluble substance milkiness is formed?
Answer:
Insoluble calcium carbonate (CaCO3) makes the solution milky.
Question 11.
What would happen when dil. HCl and dil. NaOH (same concentrations) are mixed in equal amounts? Write the equation also.
Answer:
When dil. HCl and dil. NaOH are mixed in equal amounts, neutralisation reaction takes place with formation of salt and water.
HCl (aq)+ NaOH (aq) ——–> NaCl (aq) + H2O(l)
Question 12.
What happen if we bring a match-stick near the mouth of the test tube when NaOH is added to zinc granules?
Answer:
When zinc granules is added to sodium hydroxide, hydrogn gas is liberated. When we bring a match-stick near the mouth of the test tube, this gas burns with pop sound.
Zn(s) + 2NaOH(aq) ———> Na2ZnO2(aq) + H2(g)
The match-stick gets extinguished.
Question 13.
A dilute solution of sodium carbonate was added to two test tubes — one containing dil. HCl (A) and the other containing dilute NaOH (B). What would you observe in test tubes?
Answer:
In test tube A, sodium carbonate reacts with dilute HCl to form colourless CO2 gas.
Na2CO3(aq) + 2HCl(aq) —–> 2NaCl(aq) + CO2(g) + H2O(l)
In test tube B, no reaction will take place.
Question 14.
A solution of soda ash is prepared by dissolving 1 g of it in 10 mL of distilled water. A strip of blue litmus paper A and strip of red litmus paper B is dipped in this solution. What would be the colour of strip A and B?
Answer:
The soda ash (sodium carbonate) solution is alkaline in nature. Thus, only the colour of red litmus paper changes to blue when it is dipped in soda ash solution. There is no colour change in blue litmus paper.
Question 15.
Name the product formed when aluminium metal reacts with NaOH solution.
Answer:
Sodium meta-aluminate is formed when aluminium metal reacts with NaOH solution.
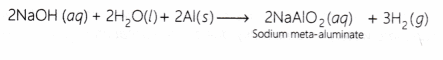
Question 16.
What will be the colour of the solution, when phenolphthalein indicator is added to sodium hydroxide solution? What happen if we add dil. HCl in this solution drop by drop?
Answer:
When phenolphthalein is added to NaOH solution, dark pink (almost purple) colour is obtained. This violet colour get disappears when dil. HCl is added in the solution slowly.
Science Lab ManualScience Practical SkillsScience LabsMath LabsMath Labs with Activity
