CBSE Class 10 Science Lab Manual – Properties of Acetic Acid
Aim
To study the following properties of acetic acid (ethanoic acid):
- Odour
- Solubility in water
- Effect on litmus
- Reaction with sodium bicarbonate
Materials Required
Test tubes, conical flask, delivery tube, glass rod, acetic acid, blue and red litmus paper or solution, distilled water, sodium hydrogen carbonate (NaHCO3), freshly prepared lime water.
Theory
An organic compound containing the carboxylic group (—COOH) is known as carboxylic acid. Acetic acid is an organic acid with the chemical formula CH3COOH. Its IUPAC name is ethanoic acid.
Acetic acid is present in vinegar. It can be obtained by wood tar distillation. It is sour in taste. It has a vinegar like smell. Vinegar, commonly called ‘Sirka’, is a dilute (4-5 percent) solution of acetic acid. Acetic acid is highly soluble in water because it gets ionised in aqueous solution and exhibits acidity.
CH3COOH (l)+ H2O (l) ——–> CH3COO–(aq)+ H3O+(aq)
The acidic property of acetic acid is due to the presence of carboxylic group (—COOH), which upon dissociation provide H+(or H3O+) ions. It turns blue litmus to red but does not affect red litmus.
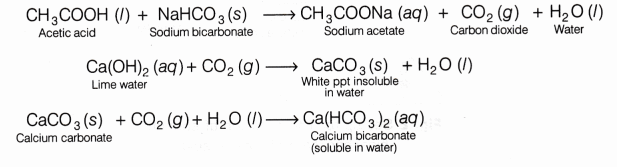
Acetic acid reacts with sodium bicarbonate and gives brisk effervescence due to the formation of CO2gas. CO2 turns lime water milky due to the formation of insoluble calcium carbonate and the milkiness disappears if excess of CO2 is passed through the solution.
Procedure
- Odour
- Take 5 ml of acetic acid in a test tube.
- Observe the colour and odour of given acid and note them in observation table.
- Solubility in Water
- Take 2 ml of water in a test tube and add 1 mL of acetic acid in it and shake it properly.
- Observe the changes occur and write it in the observation table.
- Effect on Litmus
- Take a blue litmus paper strip and put a drop of acetic acid over it using a clean glass rod as shown in Fig.1.
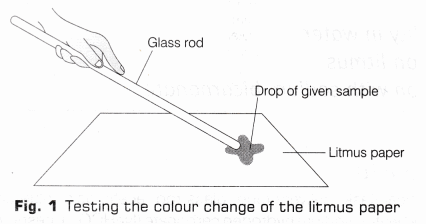
- Observe the colour change of the litmus paper and write it in the observation table.
- Repeat above steps with red litmus paper.
- Take a blue litmus paper strip and put a drop of acetic acid over it using a clean glass rod as shown in Fig.1.
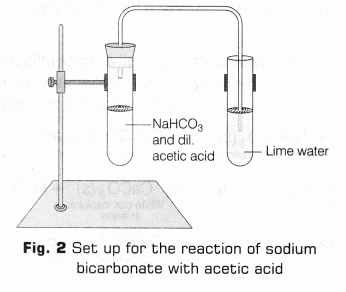
- Reaction with Sodium Bicarbonate
- Take another clean test tube and add 2 ml of acetic acid to it.
- Add a pinch of sodium bicarbonate (hydrogen carbonate) to the test tube and shake it properly.
- Hold a burning splinter near the mouth of the test tube.
- On the test tube, put a cork carrying a delivery tube, so that its other end dips in a test tube containing lime water. Allow the gas evolved to pass through lime water (Fig. 2) and write your observation in observation table.
Observation Table
| S.No. | Experiment | Observation | Inference |
| 1. | Odour Smell the sample of acetic acid taken in a test tube. | It gives a pungent smell of vinegar. | Acetic acid smells like a vinegar. |
| 2. | Solubility test Add water to a test tube containing acetic acid. | A homogeneous solution is formed. | Acetic acid is highly soluble in water. |
| 3. | Effect on litmus Put a drop of ethanoic acid over a
|
|
|
| 4. | Reaction with sodium bicarbonate
| A colourless, odourless gas is evolved which extinguish the burning splinter and turns lime water milky. On passing the gas in excess through the lime water, the milkiness disappears. | CO2 gas is liberated on the reaction between acetic acid and sodium bicarbonate. CH3COOH +NaHC03 —> |
Result
- Acetic acid has a pungent odour of vinegar.
- Acetic acid is completely soluble in water.
- It turns blue litmus paper to red.
- It evolves CO2 gas when reacts with sodium bicarbonate and sodium carbonate.
CH3COOH+ NaHCO3 ——–> CH3COONa+ H2O + CO2
2CH3COOH + Na2CO3 ———-> 2CH3COONa+CO2 + H2O
Lime water turns milky as carbon dioxide evolved above reacts with it to form insoluble calcium carbonate.
Ca(OH)2 + CO2 ———-> CaCO3 + H2O
Precautions
- Ethanoic acid should be handled carefully.
- Do not taste or touch ethanoic acid.
- Do not taste or touch sodium bicarbonate.
- Use small quantities of sodium bicarbonate to control the intensity of CO2 evolved.
- Use clean and dry test tubes.
- Do not inhale vapours of pure acetic acid directly.
- Freshly prepared lime water should be used.
Viva – Voce
Question 1.
Which gas is evolved when ethanoic acid reacts with sodium hydrogen carbonate? [NCERT]
Answer:
Carbon dioxide (CO2) gas is evolved when ethanoic acid reacts with sodium hydrogen carbonate.
CH3COOH (aq) + NaHCO3(s) ——–> CH3COONa (aq) + H2O (l) + CO2(g)
Question 2.
How will you test that the liberated gas is carbon dioxide? [NCERT]
Answer:
Carbon dioxide gas turns lime water milky and,the milkiness disappears if excess of CO2 is passed through the solution.
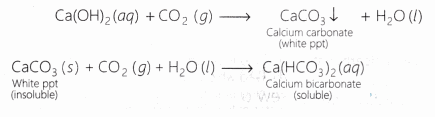
Question 3.
How will you show that ethanoic acid is acidic in nature? [NCERT]
Answer:
The acidic nature of ethanoic acid can be shown by using blue litmus paper. Dip the blue litmus paper in ethanoic acid. If it turns red, it means ethanoic acid is acidic in nature.
Question 4.
Where do you find the use of ethanoic acid in day-to-day food products? [NCERT]
Answer:
Ethanoic acid is the main constituent of vinegar which is used as a preservative.
Question 5.
What is the common name of ethanoic acid as sold in the market in the form of its dilute solution? [NCERT]
Answer:
A dilute solution of ethanoic acid is vinegar.
Question 6.
When Shweta smells acetic acid, she observes that acid smells like vinegar which is used for preserving pickles. Why does she found the odour of both substances to be similar?
Answer:
Vinegar is 5-8% aqueous solution of acetic acid. Hence, both substances have; acetic acid as a common constituent. Thus, both have same odour.
Question 7.
Acetic acid was added to small pieces of marble and the gas evolved was tested with a burning splinter. What do you observe?
Answer:
CO2 gas is evolved and when it is tested with a burning splinter then the gas does not burn but extinguishes the flame.
Question 8.
Blue and red litmus papers were dipped separately in dilute-acetic acid. What colour would you observe on the litmus papers?
Answer:
Dilute acetic acid turns blue litmus red but does not show any change/effect on red litmus paper.
Question 9.
When a white powder was mixed with acetic acid, a colourless and odourless gas was produced which turns lime water milky. What is this white powder?
Answer:
White powder is sodium hydrogen carbonate (NaHCO3) which evolve CO2 gas, turns time water milky
Question 10.
Is acetic acid an inorganic acid?
Answer:
No, acetic acid is an organic acid.
Question 11.
Which salt of carboxylic acid is commonly used as food preservative?
Answer:
Sodium benzoate is used as a food preservative.
Question 12.
What is glacial acetic acid?
Answer:
Pure (100%) ethanoic acid is called glacial acetic acid.
Question 13.
A salt X is formed and a gas is evolved when ethanoic acid reacts with sodium hydrogen carbonate. Name the salt X and the gas evolved.
Answer:
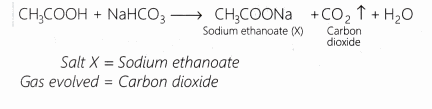
Question 14.
A student add blue litmus solution to a solution of acetic acid, it turns red. What change will be observed when a few drops of dil. HCl are added to it?
Answer:
The colour of mixture will not change, i.e. it remains red.
Question 15.
A student added acetic acid to a unknown solid A which was already kept in a test tube. A colourless and odourless gas B was evolved. The gas was passed through lime water which turned milky. Identify A and B.
Answer:
Solid A is sodium carbonate or sodium bicarbonate, and gas B is carbon dioxide.
Science Lab ManualScience Practical SkillsScience LabsMath LabsMath Labs with Activity
