CBSE Class 10 Science Lab Manual – Cleaning Capacity of Soap in Hard and Soft Water
Aim
To study the comparative cleansing capacity of a sample of soap in soft and hard water.
Materials Required
Tap water (or well water), distilled water, calcium hydrogen carbonate or calcium sulphate, soap sample, test tubes, test tube stand, glass rod, measuring cylinder (50 ml) and a measuring scale.
Theory
Hardness of water is due to the presence of the salts of calcium and magnesium (hydrogen carbonates, chlorides and sulphates) in water. Hardness is of two types, i.e. temporary hardness and permanent hardness.
Temporary hardness of water is due to the presence of calcium and magnesium bicarbonates in water and can be remove by boiling or by adding Na2CO3.
Permanent hardness of water is due to the presence of chloride and sulphates of calcium and magnesium and can be remove by using ion exchange.
When soap is added to hard water, it forms a scum by reacting with the salts of magnesium and calcium ions. This scum is insoluble and floats on the surface of water. Calcium and magnesium ions present in hard water produce curdy white precipitates of calcium and magnesium salts of fatty acids.
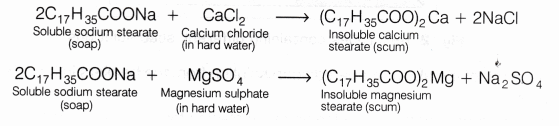
These scum creates hindrance to good washing because it adheres to fibres of the cloth as gummy mass. Therefore, the presence of calcium and magnesium salts in water precipitates the soap thereby reducing its cleansing power and hence, foaming capacity.
Note:
- When soap is shaken in soft water which does not contain Ca+2 and Mg+2 ions, lot of lather is formed which helps in cleaning clothes.
- When soap is shaken with hard water which contains Ca+2 and Mg+2 ions, very less lather is formed and cleaning of clothes does not take place.
Procedure
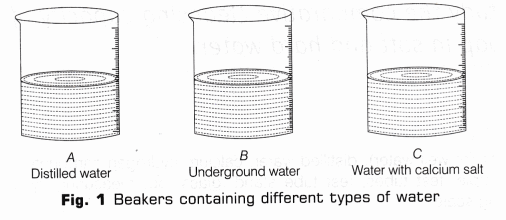
- Take three beakers and label them as A, B and C.
- Add 20 ml of distilled water in beaker A, 20 ml of underground water in beaker B and 20 ml of distilled water in beaker C (Fig. 1).
- Add 2g of calcium sulphate (or calcium hydrogen carbonate) to 20 ml of distilled water taken in C.
- Stir the contents of beaker C with the help of clean glass rod till calcium sulphate (or calcium hydrogen carbonate) dissolves in water.
- Add equal amount of weighed soap to all three beakers A, B and C.
- Stir the contents of these beakers with separate clean glass rods.
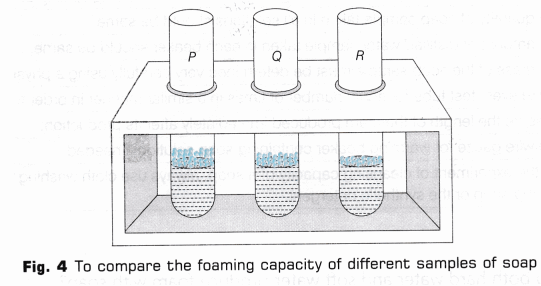
- Place three tubes in a test tube stand and label them as P, Q and R (Fig. 2).
- Pour 5 ml of the above prepared soap solution from the beakers in the corresponding test tubes.
- Take test tube P and shake it ten times by placing thumb on its mouth (Fig. 3).
- On shaking the test tube, foam or lather will be formed. Measure the length of foam produced immediately with the help of a measuring scale.
- Similarly, repeat steps 9 and 10 with the remaining two samples(Fig. 4).
Observation
- Mass of sample of soap taken in each beaker = ……….
- Volume of distilled water and underground water added in each beaker = ……….. mL
- Volume of soap sample taken in each test tube = ………….. mL
- Number of times each test tube shaken = ……………….
Calculations
| S.No. | Mixture (water + soap) | Test tube reading | Length of foam produced (cm) = Final length – Initial length | |
| Initial length before shaking (solution) (cm) | Final length after shaking (solution + foam) (cm) | |||
| 1. | Mixture of soap in distilled water (soft water in test tube P) | |||
| 2. | Mixture of soap in well water (hard water in test tube Q) | |||
| 3. | Mixture of soap in distilled water containing calcium sulphate (very hard water in test tube R) | |||
Result
- Soap solution in test tube P produces the maximum length of foam. Thus, distilled water (soft water) has the most cleansing capability.
- Soap solution in test tube 0 produces smaller length of foam as compare to test tube P. Thus, underground water has less cleansing capability than soft water.
- Soap solution in test tube R produces minimum length of foam. Thus, distilled water with calcium hydrogen carbonate has least cleansing capability.
Precautions
- Use similar soap for each water sample.
- Stir the mixture carefully while dissolving soap in water for avoiding spilling of soap solution.
- The quantity of soap sample taken in all solutions should be same.
- The amount of distilled water sample taken in each beaker should be same.
- The mass of the soap sample must be determined very carefully using a physical balance.
- Shake every test tube for equal number of times in a similar manner in order to avoid any disparity.
- Measure the length of the foam produced immediately after its production.
- Use wire gauze for warming beaker containing soap solution if needed.
- For the experiment of cleansing capacity of a soap, always use cloth washing soap and not bathing soap or the synthetic detergent.
Viva – Voce
Question 1.
Do both hard water and soft water produce foam with soap? [NCERT]
Answer:
No, only soft water produces foam with soap.
Question 2.
Why is scum formed when hard water is treated with soap? [NCERT]
Answer:
Hard water contains bicarbonate, chloride or sulphate salts of calcium and magnesium which reacts with soap to form insoluble precipitate called scum.
Question 3.
Why did we add calcium hydrogen carbonate (or calcium sulphate) to beaker C? [NCERT]
Answer:
Beaker C contains distilled water which is free ofCa+2 and Mg+2 ions. When calcium hydrogen carbonate (or calcium sulphate) is added to distilled water, the water becomes temporary hard.
On addition of soap, these ions react with soap thereby reducing lather capacity of soap by forming scum
Question 4.
Was there any difference in the length of the foam formed in test tube C having water containing calcium hydrogen carbonate (or calcium sulphate) and test tube B containing well water or underground water? [NCERT]
Answer:
Yes, test tube B contains larger length of foam while C contains smaller length of foam because test tube C contains calcium hydrogen carbonate dissolved in distilled water. This calcium hydrogen carbonate reduces the foaming capacity of soap.
Question 5.
With their prolong use, white scales get deposited in the interior of boilers and electric kettles. What is the reason for this observation? How can these scales be removed? [NCERT]
Answer:
On prolong use, scales get deposited in the interior of boilers and electric kettles due to the precipitation of CaCO3 and CaSO4. The precipitation of CaCO3 is also caused due to the thermal decomposition of bicarbonate ions. Descaling agent (like white vinegar contains acetic acid) can be used to remove scaling.
Question 6.
What do you understand by temporary and permanent hardness of water? [NCERT]
Answer:
Temporary hardness of water is due to the presence of calcium and magnesium bicarbonates in water and can be removed by boiling or by adding Na2CO3 to hard water.
Permanent hardness of water is due to the presence of chlorides and sulphates of calcium and magnesium and can be removed by using an ion exchanger.
Question 7.
What is soft water?
Answer:
The water which has few or low mineral content like calcium (Ca) or magnesium (Mg) ions and produces lather on mixing soap to it is called soft water.
Question 8.
What is hard water?
Answer:
The water that has high mineral content (like magnesium and calcium ions) and produces scum on addition of soap.
Question 9.
What is the reaction between soap molecules and ions present in hard water? [NCERT]
Answer:
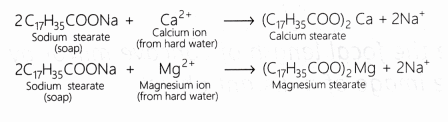
Question 10.
Which salts cause hardness in water?
Answer:
The hydrogen carbonates, chlorides and sulphates of calcium and magnesium cause hardness in water.
Question 11.
What can be used instead of soap to wash clothes in hard water?
Answer:
Synthetic detergents are used to wash clothes in hard water, as they do not produce the insoluble scum.
Question 12.
Why do detergents not produce scum with hard water?
Answer:
The calcium and magnesium salts of detergents are soluble in water. Hence, detergents can be used effectively even in hard water
Question 13.
Tanu tried to dissolve the soap in water completely but it does not dissolve and gives no lather also. What conclusion can you drawn from this observation?
Answer:
The soap does not dissolve in water completely, this suggests that the water taken by Tanu is hard, i.e. it contains salts of calcium and magnesium (generally bicarbonates, chlorides or sulphates).
Question 14.
When soap is added to hard water, a white curdy substance is observed which floats on the surface of hard water. Name this white curdy substance.
Answer:
When soap is added to hard water, it forms an insoluble white curdy substance called scum which floats on the surface of water.
Question 15.
When soap is added to ethanol solution, will micelles formed or not?
Answer:
Soap gets completely soluble in ethanol and forms a clear solution. Thus, it does not form micelles.
Question 16.
A student observed that when Na2CO3 is added to hard water, it produces lather with soap. What could you conclude from this observation?
Answer:
When Na2CO3 (sodium carbonate) is added to hard water, it reacts with calcium and magnesium ions and gets precipitated out as carbonates thereby, increasing the foaming capacity of soap and hence, soap produces lather with this type of water.
Science Lab ManualScience Practical SkillsScience LabsMath LabsMath Labs with Activity
