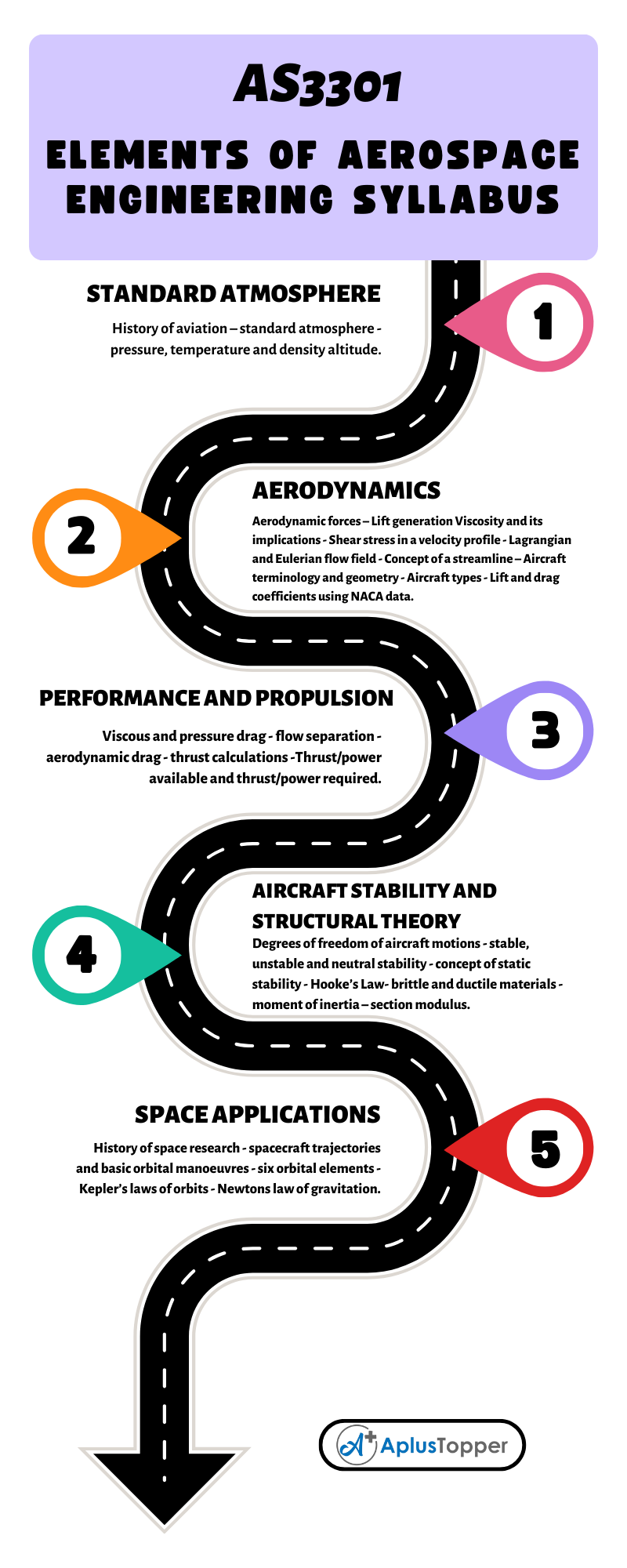
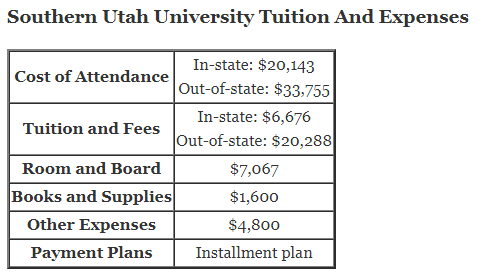
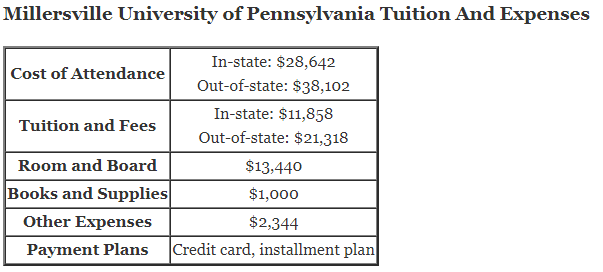
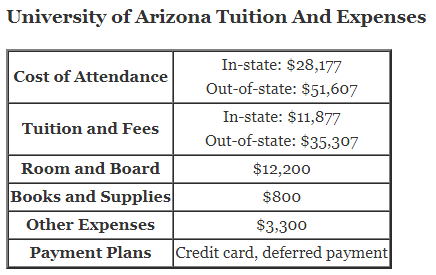
How does titration determine concentration?
How does titration determine concentration?Titration is a very useful laboratory technique in which one solution is used to analyse another solution.One of the solutions is a standard solution of known concentration and is delivered from a burette.The te…
Relationship between pH values and molarity of acids and alkalis
Relationship between pH values and molarity of acids and alkalisThe relationship between pH values and concentration of hydrogen ions is given below: Concentration of hydrogen ions increases → pH value decreasesIn an acidic solution, the concentration o…
What is meant by a neutralization reaction?
What is meant by a neutralization reaction?The reaction of an acid and a base is called a neutralisation reaction. In this reaction, the acidity of an acid is neutralised by an alkali. At the same time, the alkalinity of the alkali is neutralised by the…
How can we measure the strength of acids and alkalis?
How can we measure the strength of acids and alkalis?An acid produces hydrogen ions in aqueous solution. The acidity of a solution is a measure of the concentration of the hydrogen ions in the solution.A base produces hydroxide ions in aqueous solution. …
How do you prepare a standard solution?
How do you prepare a standard solution?Preparation of a standard solution by weighing methodA solution whose concentration is accurately known is called a standard solution.A standard solution can be prepared by weighing method in the following way. (a)…
What are the chemical properties of an acid?
What are the chemical properties of an acid?Chemical properties of acids:Acids react with reactive metals. Acid + metal → salt + hydrogenCopper and silver do not react with dilute acid. Acids react with bases. Acid + base → salt + water Acids react with …
General Properties of Bases
General Properties of BasesSome of the characteristic properties of bases are:Bases are bitter to taste a bitter taste is characteristic of all bases.Bases may or may not be soluble in water Bases that can dissolve in water are called alkalis. Some exam…
What determines a Strong Base and a Weak Base
What determines a Strong Base and a Weak BaseStrong Bases and Weak Bases : The strength of a base is determined by the amount of hydroxide ions (OH–) that the base provides when dissolved in water. Some of the bases, when dissolved in water, get almost …
What are the uses of Bases
What are the uses of BasesUses of Bases 1. Calcium Hydroxide (Slaked lime) [Ca(OH)2]It is used to neutralize the acidity in soils.It is an ingredient in whitewash and mortar.It is a component of the Bordeaux mixture used for protecting agricultural crop…
Preparation of Bases
Preparation of BasesBases can be prepared by the following methods. Methods of preparation of bases1. By the direct union of a metal with oxygen Some metals when heated in air or oxygen form the oxides of the metals. These oxides when dissolves in water…