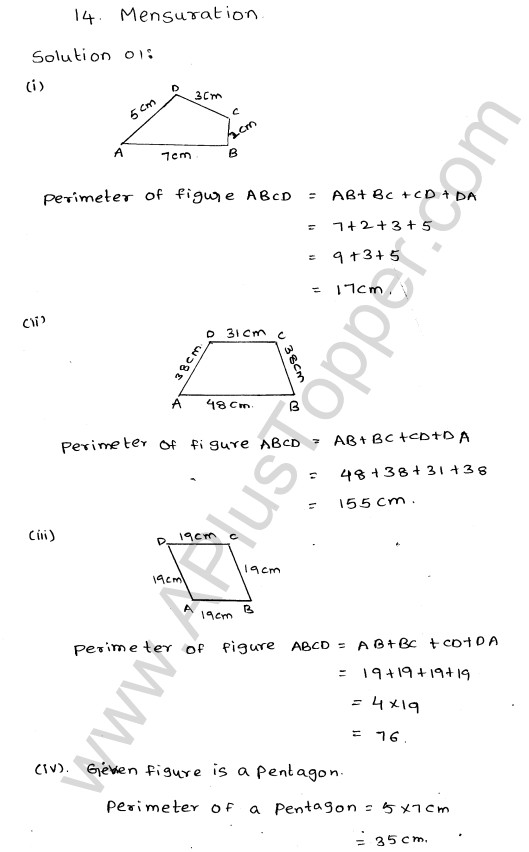
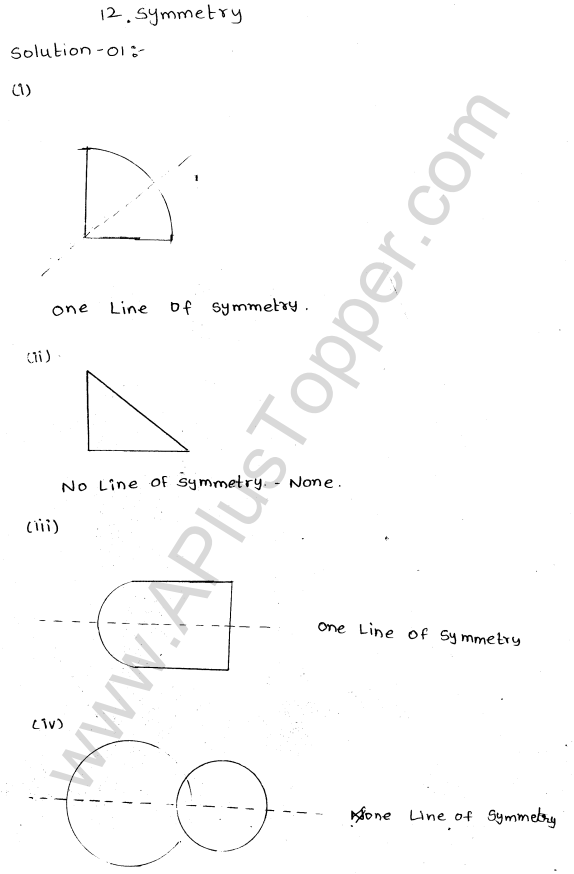
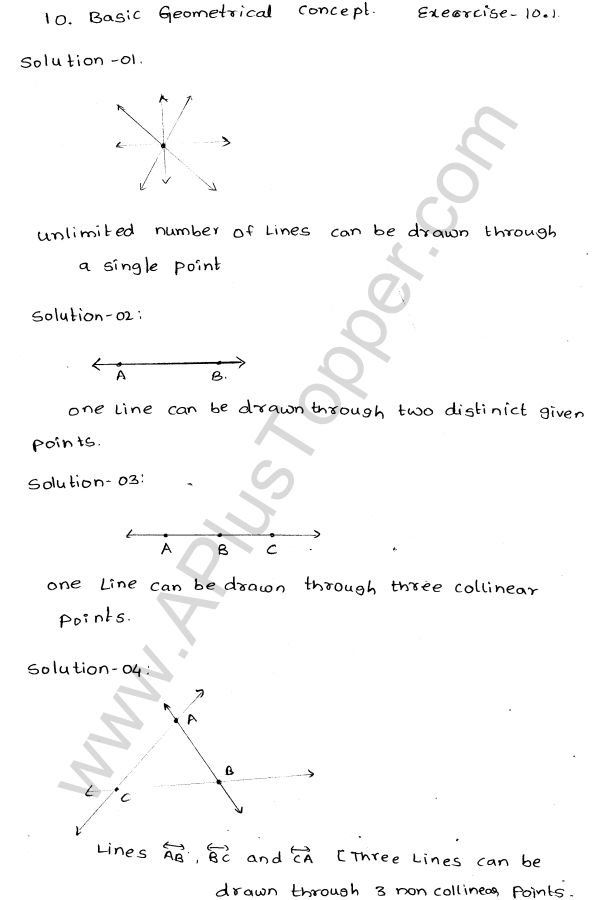
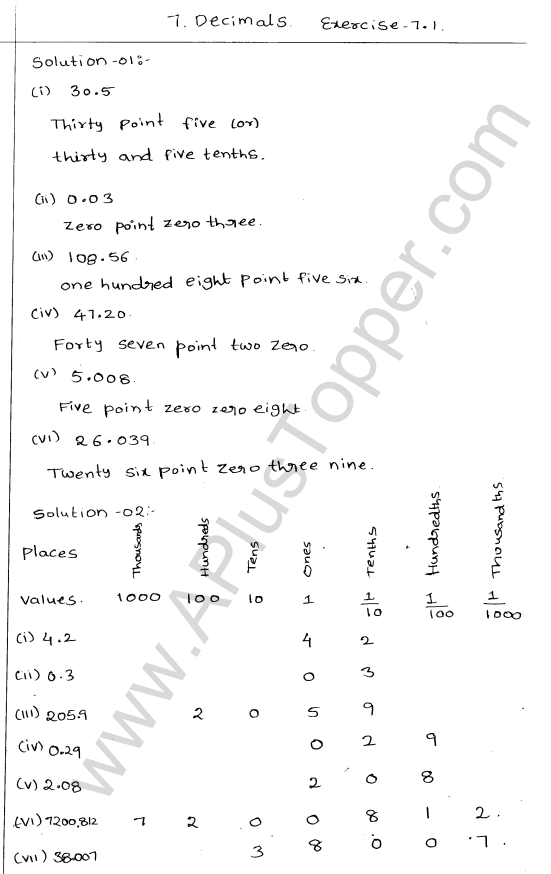
What are the Isotopes, Isobars and Isotones of an Element
What are the Isotopes, Isobars and Isotones of an ElementThe proton number of an atom determines the type of element. For example, the proton number Z = 11 is for the element sodium while Z = 12 is for magnesium. Therefore, all atoms of the same element…
How do you know the Order of Elements in a Chemical Formula
How do you know the Order of Elements in a Chemical FormulaWhat is Chemical Formula?A chemical formula is a representation of a chemical substance using letters for atoms and subscript numbers to show the numbers of each type of atoms that are present i…
Analysing the electrolysis of molten compounds
Analysing the electrolysis of molten compoundsElectrolysis is a process whereby a compound is decomposed into its constituent elements when an electric current passes through an electrolyte.Michael Faraday was a pioneer in the field of electrolysis. He …
What is the Molar Volume of a Gas at STP?
What is the Molar Volume of a Gas at STP?The Mole and the Volume of GasIt is rather tricky to find the number of moles of a gas by weighing its mass. Chemists determine the number of moles of any gas by measuring its volume. However, this cannot be done …
Why is an electrolyte able to conduct electricity while a Nonelectrolyte Cannot?
Why is an electrolyte able to conduct electricity while a Nonelectrolyte Cannot? Electrolytes and Non-electrolytes Chemical substances can be classified into electrolytes and non-electrolytes.Electrolytes are substances that can conduct electricity eithe…
What is the Relative Atomic Mass and Relative Molecular Mass of an Element?
What is the Relative Atomic Mass and Relative Molecular Mass of an Element?Relative Atomic Mass FormulaThe earlier development of relative atomic massAn atom is very tiny. Therefore, it is impossible to determine its mass by weighing. So, chemists compa…
Properties of Ionic and Covalent Compounds
Properties of Ionic and Covalent CompoundsIonic and covalent compounds differ in their properties because the particles in each of these two compounds are held together by different types of chemical bonds.Table compares and contrasts the properties of …
How would you describe the Structure of an Atom
How would you describe the Structure of an AtomAfter the discovery of electron and proton, the scientists started thinking of arranging these particles in an atom. J.J. Thomson was the first scientist to propose a model for the structure of atom. Mainly …
How do you Calculate the Molar Mass of a Substance?
How do you Calculate the Molar Mass of a Substance?Molar mass of a substance is the mass of one mole of the substance in grams. It has a unit of grams per mole (g mol-1).One mole of any substance contains 6.02 × 1023 particles. Therefore, the molar mass…
What is the Haber process used for?
What is the Haber process used for? Manufacture of ammonia in industryMost of the world supply of ammonia is manufactured through Haber process.The raw materials for the manufacture of ammonia are hydrogen gas and nitrogen gas. The ratio for the raw mate…