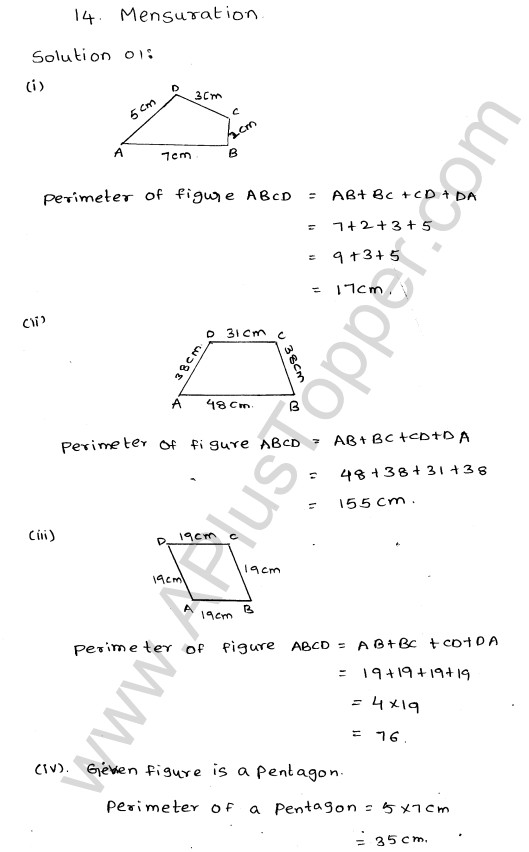
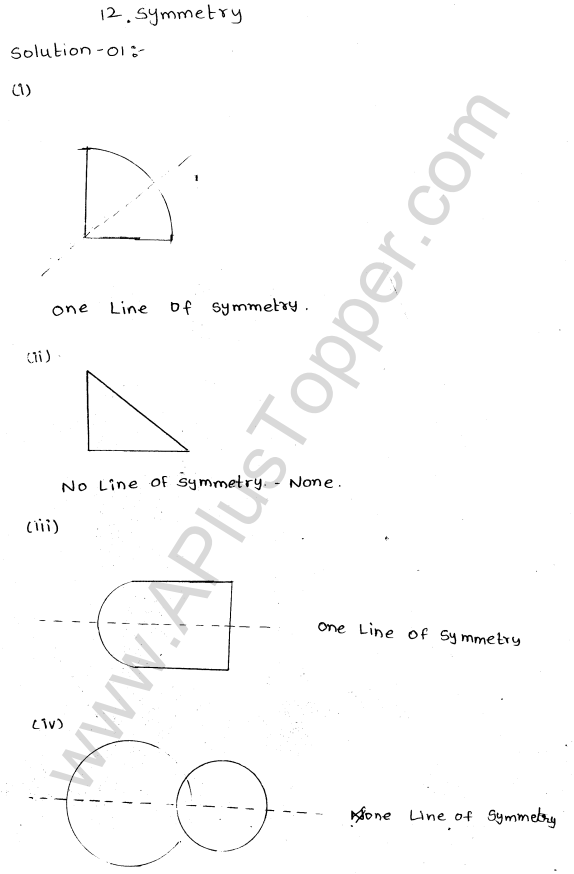
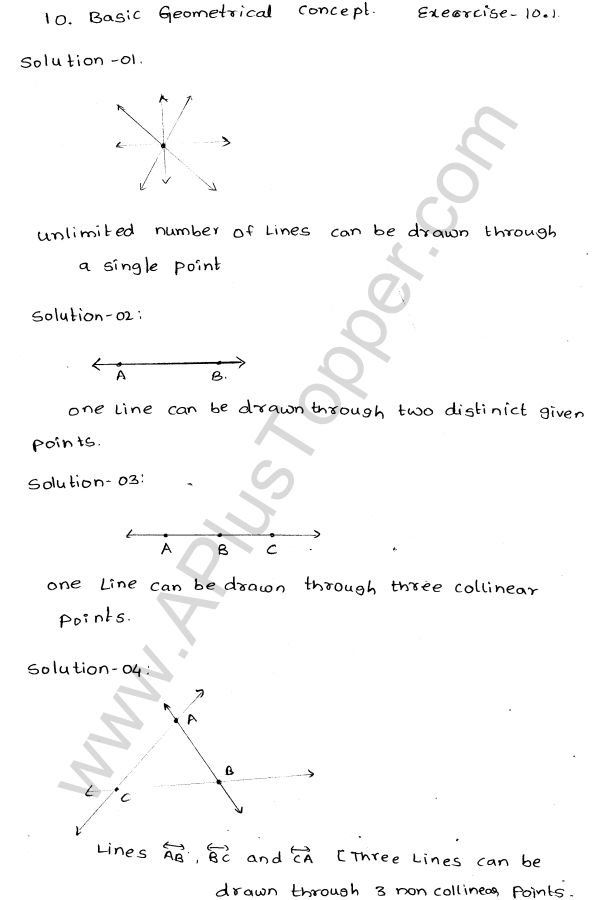
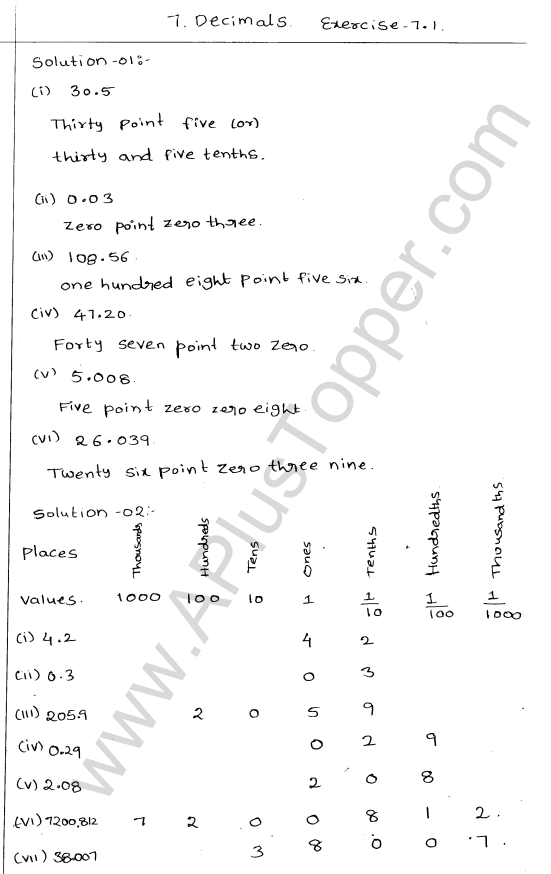
Explain the formation of ionic bonds with examples
Explain the formation of ionic bonds with examplesFormation of ionic bonds illustrated using different examplesBefore you discuss the formation of potassium chloride, calcium sulphide and magnesium fluoride, you need to find out the number of metal and …
How polymers are classified?
How polymers are classified?PolymersPolymers are long chained molecules formed by joining up many identical repeating sub-units called monomers.Polymerisation is a process by which the monomers are joining together into chain-like big molecules known as …
Explain the effect of concentration on the rate of reaction?
Explain the effect of concentration on the rate of reaction? Effect of concentration on the rate of reaction: When the concentration of a reactant increases, the rate of reaction also increases.(a) For example, two sets of experiments are carried out us…
General Properties of Salts
General Properties of SaltsSome of the characteristic properties of salts are:Melting and boiling points: Salts are mostly solids which melt as well as boil at high temperatures.Solubility in water: Salts are generally soluble in water. For example, sod…
What is the homologous series of hydrocarbons?
What is the homologous series of Hydrocarbons What do you mean by homologous series?Homologous series Organic compounds are grouped into different homologous series. Alkanes form a homologous series and so do alkenes.A homologous series is a group or fam…
What did Bohr Contribute to the Theory of an Atom
What did Bohr Contribute to the Theory of an AtomBohr’s model of an atom In 1912, Niels Bohr put forward a model to explain the structure of an atom. The main postulates of the model are: An atom is made up of three particles : electons, protons and neut…
What is the effect of a catalyst on the rate of a reaction?
What is the effect of a catalyst on the rate of a reaction? Effect of catalyst on the rate of reaction: A catalyst is a substance which can alter the rate of a chemical reaction while itself remains chemically unchanged at the end of the reaction.(a) Ca…
Describe the preparation of soluble and insoluble salts
Describe the preparation of soluble and insoluble saltsHow do you prepare a soluble salt?Preparing soluble salts of ammonium, sodium and potassium:Ammonium salts, sodium salts and potassium salts are prepared by a titration method based on neutralisatio…
Changing of iron(II) ions to iron(III) ions and vice versa
Changing of iron(II) ions to iron(III) ions and vice versaIron exhibits two oxidation numbers (a) +2 as iron(II) ion, Fe2+ (b) +3 as iron(III) ion, Fe3+An aqueous solution containing iron(II) ions, Fe2+ is pale green in colour, whereas that containing ir…
Preparation of Salts
Preparation of SaltsPreparation of salts in the laboratoryThe method used to prepare a salt depends on the solubility of the salt in water.A soluble salt can be prepared from a reaction between an acid and a metal, a base or a carbonate.Reaction 1: Acid …