How do you Calculate the Molar Mass of a Substance?
- Molar mass of a substance is the mass of one mole of the substance in grams. It has a unit of grams per mole (g mol-1).
- One mole of any substance contains 6.02 × 1023 particles. Therefore, the molar mass of a substance contains 6.02 × 1023 particles of the substance.
- The molar mass of any substance is numerically equal to its relative atomic, molecular or formula mass.
(a) This means, to measure 1 mole of atoms of any element, we only need to weigh a mass equal to its relative atomic mass in grams.
(b) Similarly, the mass of 1 mole of molecules of any molecular substance is equal to its relative molecular mass in grams.
(c) The mass of 1 mole of any ionic substance is equal to its relative formula mass in grams. - Based on Table below, 1 mole of magnesium can be measured by weighing 24 g of magnesium.
This amount of magnesium contains 6.02 × 1023 magnesium atoms.Substance Relative mass Molar mass
(g mol-1)Magnesium, Mg Ar = 24 24 Helium, He Ar = 4 4 Hydrogen gas, H2 Mr = 2(1) = 2 2 Methane, CH4 Mr = 12 + 4(1) = 16 16 Sodium chloride, NaCl Fr = 23 + 35.5 = 58.5 58.5 Zinc bromide, ZnBr2 Fr = 65 + 2(80) = 225 225 [Relative atomic mass: H, 1; He, 4; C, 12; Na, 23; g Mg, 24; Cl, 35.5; Zn, 65; Br, 80]
- The mass of any number of moles of a substance or vice versa can be calculated easily using the following relationship.
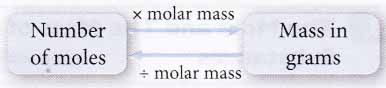
- Equal numbers of moles of substances always contain the same number of particles even though they differ in mass and size.

Figure: Both blocks of magnesium and iron contain 0.5 x 6.02 x 1023 atoms. [Relative atomic mass: Mg, 24; Fe,56] - For this reason, we can compare the number of particles in substances by comparing the number of moles of the substances.
People also ask
- What is the Relative Atomic Mass and Relative Molecular Mass of an Element?
- What is One Mole and How many Particles are in a Mole?
- What is the Molar Volume of a Gas at STP?
- How do you know the Order of Elements in a Chemical Formula
- What is Empirical and Molecular Formula?
- How do you Write a Chemical Equation?
Molar Mass of a Substance Problems with Solutions
1. Find the mass of the following.
(a) 0.5 mole of copper
(b) 1.5 moles of carbon dioxide
[Relative atomic mass: C, 12; O, 16; Cu, 64]
Solution:
(a) Relative atomic mass of copper = 64
So, the molar mass of copper = 64 g mol-1
Mass of 0.5 mole of copper = number of moles x molar mass
= 0.5 x 64 = 32g
(b) Relative molecular mass of carbon dioxide, CO2 = 12 + 2(16) = 44
So, the molar mass of CO2 = 44 g mol-1
Mass of 1.5 moles of CO2 = number of moles x molar mass
= 1.5 x 44 = 66g
2. Find the number of moles of sodium hydroxide with the mass of 2.0 g.
[Relative atomic mass: H, 1; O,16; Na, 23]
Solution:
Relative formula mass of sodium hydroxide, NaOH = 23+16+1 = 40
So, the molar mass of NaOH = 40 g mol-1
Therefore, the number of moles of NaOH
= mass of NaOH ÷ molar mass
= 2/40
= 0.05 mol
3. What is the number of moles of calcium nitrate in 49.2 g of calcium nitrate, Ca(NO3)2?
[Relative atomic mass: O, 16; Ca, 40; N, 14]
A. 0.30 mol
B. 0.36 mol
C. 0.45 mol
D. 0.48 mol
Solution:
Relative formula mass of Ca(NO3)2 = 40 + 2[14 + 3(16)] = 164
So, molar mass of Ca(NO3)2 = 164 g mol-1
Number of moles of Ca(NO3)2 = Mass of Ca(NO3)2 ÷ molar mass of Ca(NO3)2
= 49.2/164
= 0.3 mol
Answer: A
4. What is the mass of gold that has the same number of atoms as in 4 g of oxygen?
[Relative atomic mass: O, 16; Au, 197]
Solution:
Number of moles of oxygen atoms, O = mass of O ÷ molar mass of O
= 4/16 = 0.25 mol
0.25 mole of gold will have the same number of atoms with 0.25 mole of oxygen.
Therefore, the mass of gold, Au
= number of moles of Au x molar mass of Au
= 0.25 x 197
= 49.25 g
5. What is the mass of hydrogen gas that has twice the number of molecules as in 1.6 g of oxygen gas?
[Relative atomic mass: H, 1; O,16]
Solution:
Relative molecular mass of oxygen gas, O2 = 2(16) = 32
Therefore, the molar mass of O, = 32 g mol-1
Number of moles of O2 = mass of O2 ÷ molar mass of O2
= 1.6/32 = 0.05 mol
Thus, the number of moles of hydrogen gas, H2 that contains twice the number of molecules in 0.05 mole of O2
= 2 x 0.05 mol = 0.1 mol
Mass of 0.1 mole of H2
= number of moles of H2 x molar mass of H2
= 0.1 x 2(1) = 0.2 g
6. How many times more is the number of atoms in 7 g of nitrogen gas compared to that in 7 g of iron? [Relative atomic mass: N, 14; Fe, 56]
Solution:
Here, we need to compare the number of moles of atoms in both substances.
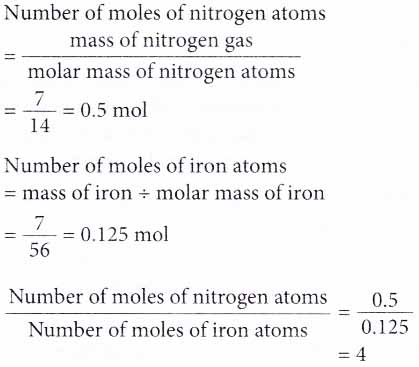
Therefore, the number of atoms in 7 g of nitrogen gas is 4 times more than that in 7 g of iron.
