Binding of Carbon with other Elements
We have understood that the compounds of carbon and hydrogen are called hydrocarbons.
Practical observations reveal that carbon forms compounds not only with atoms of hydrogen but also with atoms of other elements like oxygen, nitrogen, sulphur, phosphorus, halogens etc.
Let us see the compounds of carbon with other elements.
Carbon compounds with C, H, X.
• Compounds containing C, H, X where ‘X’ represents halogens (Cl, Br, I atoms).
Eg: CH3Cl, CH3– CH2–Br, CH2Cl – CH2I, CH3 – CHCl2
These are known as halogen derivatives of hydrocarbons or halo hydrocarbons.
Carbon compounds with C, H, O.
• Compounds containing C, H, O are of different types:
Alcohols
If a hydrogen atom of H2O molecule is replaced by ‘R’ we get alcohols R-OH.
The hydrocarbons that contain –OH group are called alcohols. Observe the following examples:
CH3OH, CH3CH2OH, CH3-CHOH-CH3 etc.
General formula of alcohols is R – OH where ‘R’ is alkyl group, CnH2n+1.
Aldehydes
The hydrocarbons with functional group –CHO are called aldehydes.
Observe the following examples:
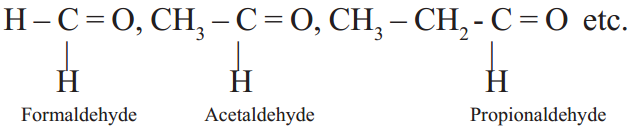
General formula of aldehydes is R – CHO, where R = alkyl group or hydrogen and -CHO is functional group.
Ketones
The hydrocarbons with ![]() functional group are called ketones.
functional group are called ketones.
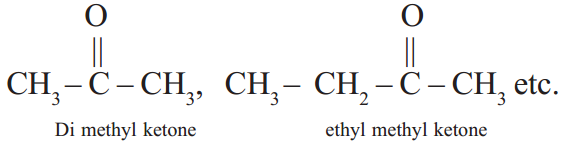
![]() group is known as Ketone group(common system)
group is known as Ketone group(common system)
General formula of Ketones is 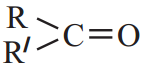
R and R’ are alkyl groups which may be same or different.
Carboxylic acids
The general molecular formula of carboxylic acid is R – COOH, where R is an alkyl group or H atom.
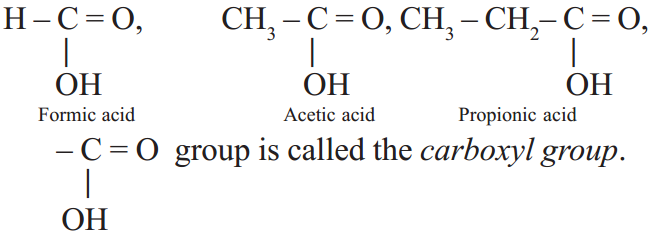
Ethers
Ethers are carbon compounds related to H2O in such a way that both hydrogen atoms are replaced by two alkyl groups which may be same or different.
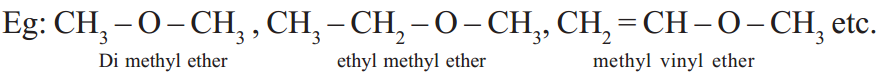
Esters
These compounds are derivatives of carboxylic acids. If the hydrogen atom of – COOH gets replaced by ‘R’, the alkyl group esters are obtained.
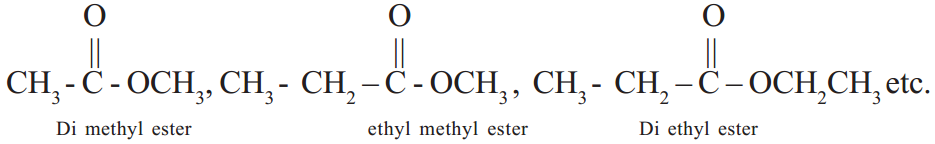
Compounds containing C, H, N
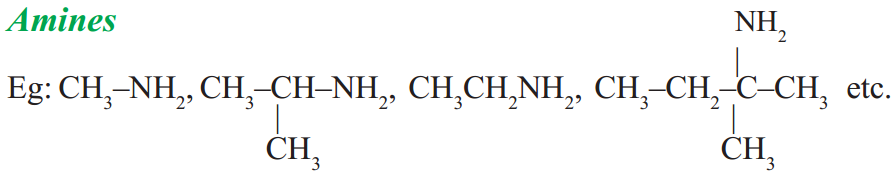
– NH2 group is called amine group. We may compare amines to NH3 as we have done ROH and R – O – R’ to H2O.
If one hydrogen atom is replaced from NH3 by an alkyl group we get the so called primary amines. If two hydrogen atoms of NH3 are replaced by two alkyl groups (same or different) we get secondary amines and if all the three hydrogen atoms are replaced by the same or different alkyl groups we get tertiary amines.
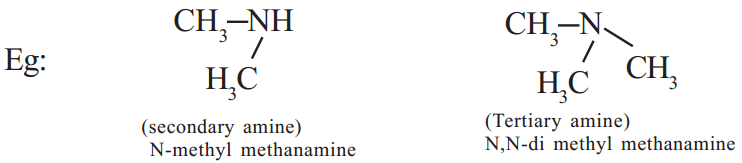
Read More:
- Homologous Series of Hydrocarbons
- Allotropes of Carbon
- Catenation in Carbon
- Classification of Hydrocarbons
- sp3 Hybridized Carbon atom
- Hybridization of the Carbon atoms in Acetylene
- Versatile Nature of Carbon
- What are the Characteristics of Compounds
- Chemical Properties of Carbon Compounds
- Nomenclature of Carbon Compounds
