Applications of exothermic and endothermic reactions in everyday life
Application of exothermic and endothermic reactions:
- The principle of exothermic and endothermic reactions is applied in instant cold packs and hot packs which are used to treat sports injuries.
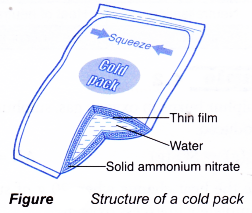
- Instant cold packs have separate compartments of water and solid ammonium nitrate placed in a plastic bag. The compartments are normally separated by a thin film.
- When the outer bag is squeezed, the thin film between the two compartments is broken. The solid ammonium nitrate dissolves in the water endothermically to provide instant coldness.
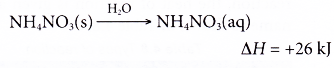
- Heat is absorbed from the surroundings such as the injured area of the athlete’s body.
- Other substances that can be used in a cold pack:
(i) Ammonium chloride
(ii) Potassium nitrate
(iii) Sodium thiosulphate
- A hot pack has a similar structure as the cold pack. It is a plastic bag containing separate compartments of water and anhydrous calcium chloride.
- When the outer bag is squeezed, the thin film between the two compartments is broken. The anhydrous calcium chloride dissolves in the water exothermically to release heat.
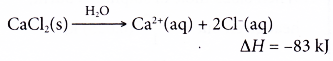
- Other substances that can be used in a hot pack:
(i) Anhydrous magnesium sulphate
(ii) Anhydrous copper(II) sulphate
(iii) Calcium oxide
- A reusable hot pack uses sodium acetate crystallisation and resolution system. By bending the metal disc in the bag, the sodium acetate crystallises and gives out heat. Placing the bag in boiling water redissolves the sodium acetate crystals, and thus the hot pack can be reused.
- Solid sodium hydroxide which is sold in the market as lye, is a common drain cleaner. Dissolving lye in water is an exothermic process and the heat liberated may melt grease, allowing it to be flushed from a clogged drainpipe.
People also ask
- How can energy be changed in a chemical reaction?
- How does the energy level diagram show this reaction is exothermic?
- What is enthalpy of reaction?
- What is heat of precipitation?
- What is heat of displacement?
- What is the enthalpy of neutralization?
- What is the heat of combustion?
- Why is energy released when a bond is formed?
- What is meant by the calorific value of a fuel?
