What is alloy metal?
- An alloy is a mixture of two or more elements with a certain fixed composition in which the major component must be a metal.
- In the process of making alloys, one or more foreign elements are added to a molten metal. Thus, the positions of some metal atoms are replaced by the atoms of foreign metal which may be bigger or smaller.
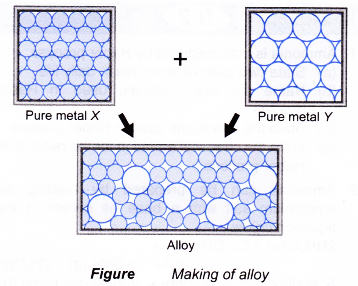
- These foreign atoms of different sizes disrupt the orderly arrangement of the metals. Thus, the properties of the pure metal are improved.
- Alloys are stronger, harder, more resistant to corrosion, have a better finish and more lustrous than their pure metal.
- Most alloys are mixture of metals. Some alloys may contain mixture of a metal and a non-metal.
Example:
(a) Brass is a mixture of copper and zinc.
(b) Steel is a mixture of iron and carbon.
(c) Stainless steel is a mixture of iron, carbon and chromium. - By changing the percentage composition of the metals, the properties of the resulting alloy can be altered.
Why are alloys are made?
Aims of making alloys:
The three aims of making alloy are:
(a) To increase the strength and hardness of a pure metal
(b) To increase the resistance to corrosion of a pure metal
(c) To improve the appearance of a pure metal
- To increase the strength and hardness of a pure metal.
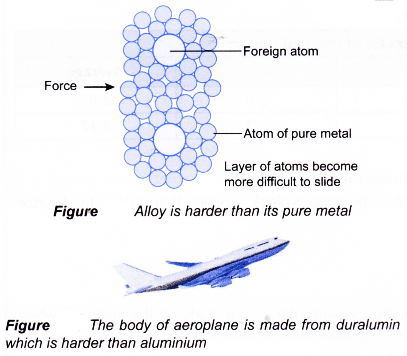
(a) During alloying, a small amount of atoms of other element is added to a molten pure metal. When the alloy becomes solid, the positions of some atom of the pure metal are replaced by atoms of other element of different sizes.
(b) The presence of these foreign atoms of different sizes disrupts the orderly arrangement of the atoms in the pure metal.
(c) This reduces the layers of atoms from sliding over one another and making alloys harder and stronger than pure metals.
(d) For example, when carbon atoms are added to iron to form steel, the carbon atoms which are smaller size than iron atoms disrupt the orderly arrangement of iron atoms making it more difficult for the layers of atoms to slide over one another. This makes steel harder than pure iron.
- To increase the resistance to corrosion of a pure metal
(a) Most metal corrode readily when exposed to air. This is because they react with oxygen and water vapour in the air.
(b) Alloying can prevent metals from corrosion. This is because alloying helps to prevent the formation of oxide layer on the surface of the metal.
(c) For example, carbon, chromium and nickel are added to iron to make stainless steel. Cutlery made from stainless steel does not corrode easily.

- To improve the appearance of a pure metal
(a) Metals have lustrous surface. However, the formation of dull metal oxide on the surface of a metal makes it quickly lose its shine.
(b) Alloying helps to keep the metal surface shiny as it prevents the formation of metal oxide.
(c) For example, atoms of antimony and copper are added to tin, making pewter to have more lustrous surface than tin.

People also ask
- How polymers are classified?
- How is glass produced from raw materials?
- How ceramics are made?
- How is a composite material made?
List of alloys and their composition and uses
Composition, properties and uses of alloys:
- Today many alloys have been discovered and are being improved by altering their percentage composition.
- The use of each different type of alloys depends on the properties of the alloy.
- Table shows the composition, properties and uses of some alloys.
| Alloy | Composition | Properties | Use |
| Bronze | 80% copper, 20% tin | Hard, strong, does not corrode easily, shiny surface | Medals, statues, monuments, art materials |
| Brass | 70 % copper, 30% zinc | Harder than copper, shiny surface | Musical instruments, kitchenware, door knobs, bullet cases, decorative ornaments, electric parts. |
| Cupro-nickel | 75% copper, 25% nickel | Beautiful surface, shiny, hard, does not corrode easily | Coin |
| Steel | 99% iron, 1% carbon | Hard, strong | Buildings, bridges, body of cars, railway track |
Stainless steel | 74% iron, 8% carbon, 18% chromium | Shiny, strong, does not rust | Cutlery, sinks, pipes, surgical instruments |
| Duralumin | 93% aluminium, 3% copper, 3% magnesium, 1% manganese | Light, strong | Body of air crafts, bullet trains, racing bicycles |
| Pewter | 96% tin, 3% copper, 1% antimony | Shiny, strong, does not corrode | Art objects, souvenirs |
| Solder | 50 % tin, 50 % lead | Hard, shiny, low melting point | Solder for electric wires and metal |
| 9-carat gold | 37.5% gold, 51.5% copper, 11 % silver | Shiny, strong, does not corrode | Jewelry |
Is alloy harder than its pure metal experiment
Aim: To investigate whether alloy is harder than its pure metal.
Problem statement: Is alloy harder than its pure metal?
Hypothesis: Bronze is harder than copper.
Variables:
(a) Manipulated variable : Different types of materials (copper and bronze)
(b) Responding variable : Diameter of the dent
(c) Controlled variables : Diameter of steel ball bearing, height of the weight, mass of the weight
Operational definition: If the diameter of the dent is smaller, then the material is harder.
Materials: Copper block, bronze block, cellophane tape.
Apparatus: Retort stand and clamps, 1 -kg weight, metre rule, steel ball bearing, thread.
Procedure:
- A steel ball bearing is taped onto a copper block using cellophane tape.
- A 1-kg weight is hung at a height of 50 cm above the copper block as shown in Figure.
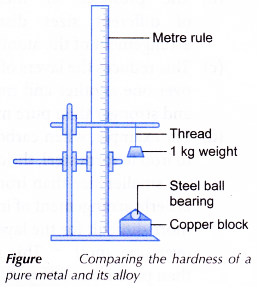
- The weight is allowed to drop onto the ball bearing.
- The diameter of the dent made by the ball bearing on the copper block is measured.
- Steps 1 to 4 are repeated twice on other parts of the copper block in order to obtain an average value for the diameter of dents formed.
- Steps 1 to 5 are repeated using a bronze block to replace the copper block with other factors remain unchanged.
- The readings are recorded in the table below.
Results:
| Metal block | Diameter of the dent (mm) | |||
| 1 | 2 | 3 | Average | |
| Copper | 2.9 | 2.8 | 2.9 | 2.87 |
| Bronze | 2.1 | 2.2 | 2.2 | 2.17 |
Discussion:
- The smaller the diameter of the dent, the harder and stronger is the material.
- The average diameter of the dents made on the surface of the copper block is bigger than that of the bronze block.
- Based on the results, bronze is harder than copper.
Conclusion:
The hypothesis is accepted.
The operational defination of hardness in this experiment is the measure of the dent made on the materials when 1 kg-weight from a height of 50 cm is dropped on the ball bearing tapped to the material.
The smaller the diameter of the dent, the harder is the material.
Does iron rust faster than steel experiment
Aim: To investigate whether iron rust faster than steel, steel rusts faster than stainless steel.
Problem statement: Does iron rust faster than steel? Does steel rust faster than stainless steel?
Hypothesis: Iron rusts faster than steel, and steel rusts faster than stainless steel.
Variables:
(a) Manipulated variable : Different types of nails
(b) Responding variable : Intensity and amount of blue colour
(c) Controlled variables : Size of nails, concentration of solutions used, duration for rusting
Operational definition: The more intense the blue colour formed, the higher is the rate of rusting.
Materials: Iron nail, steel nail, stainless steel nail, jelly solution, potassium hexacyanoferrate(lll) solution, water,
sandpaper.
Apparatus: Test tubes, test tube rack.
Procedure:
- The nails are rubbed using sandpaper to remove the rust from the surfaces of the nails.
- The iron nail is placed in test tube A, the steel nail in test tube B and the stainless steel nail in test tube C.
- A 5% jelly solution is preparedly adding 5 g of jelly into 100 cm3 of boiling water. A few drops of potassium hexacyanoferrate(lll) solution are then added to the jelly solution.
- The hot jelly solution is poured into the three test tubes until all the nails are fully immersed.
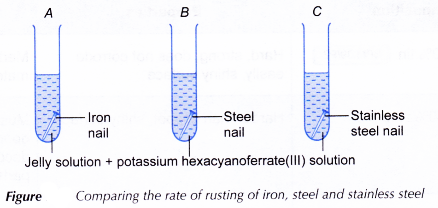
- The test tubes are placed in a test tube rack and left aside for three days. The intensity of the blue colour is observed.
- All observations are recorded in the table below.
Observations:
| Test tube | Intensity of blue colour | Inference |
| A | Very high | Rusting occurs very fast. |
| B | Low | Rusting occurs slowly. |
| C | Nil | No rusting occurs. |
Discussion:
- When iron rusts, each iron atom loses two electrons to form an iron(ll) ion, Fe2+.
Fe(s) → Fe2+(aq) + 2e–(aq) - Potassium hexacyanoferrate(lll) solution is added to the jelly solution as an indicator to detect iron(ll) ions.
- When there is iron(ll) ion, potassium hexacyanoferrate(lll) solution will form dark blue colouration.
- The higher the intensity of the blue colour, the higher is the rate of rusting.
- Solidified jelly solution is used to trap and seethe blue colouration clearly. This is because diffusion occurs the slowest in solids.
- Based on the observations, iron rusts faster than steel. Stainless steel does not rust.
- The nail made from stainless steel does not rust. This is because this nail is an alloy of iron with carbon, chromium and nickel.
- The nail made from steel will rust slowly. The presence of carbon atoms will make the steel stronger than iron but does not prevent it from rusting.
- Rusting of iron is an example of corrosion. When corrosion occurs, the metal loses electrons to form metal ion.
Conclusion:
Iron rusts faster than steel, steel rusts faster than stainless steel. The hypothesis is accepted.
In this experiment, the operational defination of rusting is the formation of dark blue colour when the different nails are immersed in jelly solution containing potassium hexacyanoferrate(lll) solution.
The more the dark blue colour formed, the higher is the rate of rusting.
