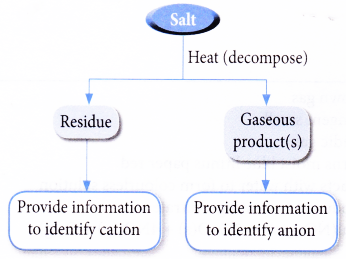
Action of Heat on Salts
- Heating a salt may cause it to decompose. The decomposition may result in
(a) a colour change
(b) evolution of a gas
(c) liberation of water vapour - Gases such as carbon dioxide, sulphur dioxide, nitrogen dioxide, ammonia or oxygen can be evolved. By identifying the gas or gases liberated, it is possible to pinpoint the anion present in the salt.
- Examination of the residue can provide information to identify the cation in the salt.
Action of Heat on Carbonate Salts
- Most metal carbonates decompose on heating to produce metal oxides and carbon dioxide gas.
Metal carbonate → metal oxide + carbon dioxide
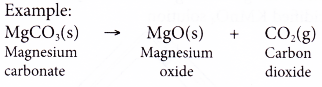
- When the carbon dioxide gas is bubbled through limewater, it will turn the limewater milky.
Table shows the action of heat on carbonate salts.
| Carbonate salt | Action of heat |
| Potassium carbonate Sodium carbonate | Do not decompose |
| Calcium carbonate Magnesium carbonate Aluminium carbonate Zinc carbonate Iron(III) carbonate Lead(II) carbonate Copper(II) carbonate | Decompose to produce metal oxide and carbon dioxide Metal carbonate → metal oxide + carbon dioxide For example, CaCO3(s) → CaO(s) + CO2(g) |
| Silver carbonate | Decomposes to produce metal, oxygen and carbon dioxide |
| Ammonium carbonate | Decomposes to produce ammonia, water and carbon dioxide |
People also ask
- Classification of Salts
- General Properties of Salts
- Uses of different salts in daily life
- Preparation of Salts
- Describe the preparation of soluble and insoluble salts
- Qualitative Analysis of Salts
- Test for Cations and Anions in Aqueous Solutions
- Constructing ionic equations using the continuous variation method
- What is stoichiometry and why is it used in chemistry?
Action of Heat on Carbonate Salts Experiment
Aim: To investigate the action of heat on carbonate salts.
Materials: Limewater, sodium carbonate, magnesium carbonate, calcium carbonate, zinc carbonate, lead(II) carbonate, copper(II) carbonate and potassium carbonate.
Apparatus: Test tubes, tongs, spatula, Bunsen burner and stopper with delivery tube.
Procedure:
- About two spatulaful of copper(II) carbonate are placed in a test tube.
- The colour of the carbonate salt is noted.
- The test tube is stoppered with a delivery tube dipping into limewater as shown in Figure.
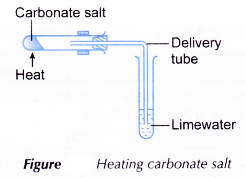
- The carbonate salt is then heated strongly.
- Any changes that occur to the limewater and also the colour of the residue when it is hot and when it is cold are recorded.
- Steps 1 to 5 are repeated using each of the carbonate salts listed in Table to replace the copper(II) carbonate.
Observations:
| Carbonate salt | Colour of salt before heating | Colour of residue | Effect on limewater | |
| Hot | Cold | |||
| Copper(II) carbonate | Green | Black | Black | Limewater turns milky. |
| Sodium carbonate | White | – | – | No change. |
| Potassium carbonate | White | – | – | No change. |
| Calcium carbonate | White | White | White | Limewater turns milky. |
| Magnesium carbonate | White | White | White | Limewater turns milky. |
| Zinc carbonate | White | Yellow | White | Limewater turns milky. |
| Lead(II) carbonate | White | Brown | Yellow | Limewater turns milky. |
Discussion:
- Alkali metal carbonates such as sodium carbonate and potassium carbonate are stable to heat.
- Most metal carbonates decompose on heating to produce metal oxides and liberate carbon dioxide gas.
- The carbon dioxide gas forms a white precipitate with limewater, making the limewater milky.
Conclusion:
Heating a metal carbonate will decompose it into a metal oxide and liberate carbon dioxide. Group 1 metal carbonates are not decomposed by heat.
Action of Heat on Nitrate Salts
- Nitrate salts also undergo decomposition on heating.
- Most metal nitrates decompose to produce a metal oxide, nitrogen dioxide and oxygen.

- Sodium nitrate and potassium nitrate decompose to produce nitrite salts and oxygen.
- Nitrogen dioxide is a brown gas. It is an acidic gas that turns moist blue litmus paper red. Hence, dissolving it in water produces a colourless acidic solution.
2NO2(g) + H2O(l) → HNO2(aq) + HNO3(aq) - The colourless oxygen gas rekindles a glowing wooden splint.
Table: Action of heat on nitrate salts
| Nitrate salt | Action of heat |
| Potassium nitrate Sodium nitrate | Decompose to produce a nitrite salt and oxygen 2KNO3(s) → 2KNO2(s) + O2(g) 2NaNO3(s) → 2NaNO2(s) + O2(g) |
| Calcium nitrate Magnesium nitrate Aluminium nitrate Zinc nitrate Iron(II) nitrate Iron(III) nitrate Lead(II) nitrate Copper(II) nitrate | Decompose to produce metal oxide, nitrogen dioxide and oxygen |
| Silver nitrate | Decomposes to produce metal, nitrogen dioxide and oxygen 2AgNO3(s) → 2Ag(s) + 2NO2 (g) + O2(g) |
| Ammonium nitrate | Decomposes to produce nitrous oxide and water NH4NO3(s) → N2O(g) + 2H2O(l) |
Action of Heat on Nitrate Salts Experiment
Aim: To investigate the action of heat on nitrate salts.
Materials: Sodium nitrate, magnesium nitrate, calcium nitrate, zinc nitrate, lead(II) nitrate, copper(II) nitrate, potassium nitrate, iron(III) nitrate, iron(II) nitrate, blue litmus paper and wooden splint.
Apparatus: Test tubes, tongs, spatula and Bunsen burner.
Procedure:
- About two spatulaful of copper(II) nitrate are placed in a test tube.
- The colour of the nitrate salt is noted.
- The nitrate salt is then heated strongly as shown in Figure.
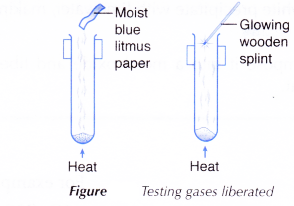
- The gases liberated are tested by
(a) lowering a glowing wooden splint into the test tube.
(b) bringing a piece of moist blue litmus paper to the mouth of the test tube. - The colour of the residue when it is hot and when it is cold are recorded.
- Steps 1 to 5 are repeated using each of the nitrate salts listed in Table to replace the copper(II) nitrate.
Observations:
| Nitrate salt | Colour of salt before heating | Colour of residue | Tests for gases evolved | |||
| Hot | Cold | Colour of gas | Glowing splint | Blue litmus paper | ||
| Copper(II) nitrate | Blue | Black | Black | Brown gas and colourless gas | Rekindles | Turns red |
| Sodium nitrate | White | White | White | Colourless | Rekindles | No change |
| Potassium nitrate | White | White | White | Colourless | Rekindles | No change |
| Calcium nitrate | White | White | White | Brown gas and colourless gas | Rekindles | Turns red |
| Magnesium nitrate | White | White | White | Brown gas and colourless gas | Rekindles | Turns red |
| Zinc nitrate | White | Yellow | White | Brown gas and colourless gas | Rekindles | Turns red |
| Iron(II) nitrate | Green | Black | Black | Brown gas and colourless gas | Rekindles | Turns red |
| Iron(III) nitrate | Brown | Brown | Brown | Brown gas and colourless gas | Rekindles | Turns red |
| Lead(II) nitrate | White | Brown | Yellow | Brown gas and colourless gas | Rekindles | Turns red |
Discussion:
- When nitrate salts are heated, they decompose to liberate nitrogen dioxide and oxygen.
- Only sodium nitrate and potassium nitrate decompose to liberate oxygen.
- Nitrogen dioxide is a brown gas that turns moist blue litmus paper red.
- Oxygen is a colourless gas that relights a glowing wooden splint.
Conclusion:
Most metal nitrates decompose to produce a metal oxide, nitrogen dioxide and oxygen except sodium nitrate and potassium nitrate which decompose to produce nitrite salts and oxygen.
Action of Heat on Sulphate Salts
- The normal sulphate salts are more stable to heat compared to the carbonates and nitrates.
- Group 1 metal sulphates such as sodium sulphate and potassium sulphate do not decompose on heating. Group 2 metal sulphates such as calcium sulphate also do not decompose when heated.
- The sulphates of heavy metals are decomposed into metal oxides and sulphur trioxide when heated.
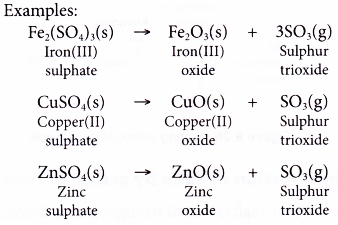
- Sulphur trioxide is a typical acidic oxide and dissolves in water to form sulphuric acid.
SO3(g) + H2O(l) → H2SO4(aq) - An exceptional case is iron(II) sulphate because it also forms sulphur dioxide gas.
2FeSO4(s) → Fe2O3(s) + SO3(g) + SO2(g)
The green crystals of iron(II) sulphate turn into a brown solid of iron(III) oxide. - Ammonium sulphate sublimes when first heated. Further heating decomposes the salt into ammonia and hydrogen sulphate.
(NH4)2SO4(S) – 2NH3(g) + H2SO4(g)
Action of Heat on Chloride Salts
- Chloride salts are stable to heat except ammonium chloride.
- Initial heating of ammonium chloride causes the salt to sublime.
NH4Cl(S) → NH4Cl(g) - On further heating, decomposition takes place to produce ammonia and hydrogen chloride.
NH4Cl(g) → NH3(g) + HCl(g) - When ammonium chloride is heated in a test tube, the lighter ammonia gas will emerge first and turn a piece of moist red litmus paper blue. Hydrogen chloride, coming up next, will change the litmus paper from blue back to red.
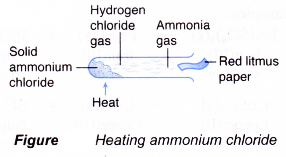
Identification of salts by action of heat
- When a salt is heated strongly, it may decompose. One or more gases may be liberated.
- Each gas can be identified by
- noting its colour.
- testing it with moist blue or red litmus paper.
- testing it with limewater.
- testing it with glowing wooden splint.
- testing it with acidified potassium dichromate(VI) solution or acidified potassium manganate(VII) solution.
- The colour of the residue when hot and cold must be noted to help in the identification of the salt.
- The following table shows how to identify salts P, Q, R, S and T through gases liberated by the action of heat.
| Test | Observation | Inference |
| Heat P in a test tube. Identify the gas/gases given off. |
|
|
| Heat Q in a test tube. Identify the gas/gases given off. |
|
|
| Heat R in a test tube. Identify the gas/gases given off. |
|
|
| Heat S in a test tube. Identify the gas/gases given off. |
|
|
| Heat Tin a test tube. Identify the gas/gases given off. |
|
|
