Which acid is used for coagulating rubber from latex?

Coagulation process of latex:
- The milky fluid obtained from tapped rubber trees is called latex.
- It consists of an aqueous suspension of colloidal rubber particles.
- Each rubber particle is made up of rubber polymers covered by a layer of protein membrane.
- Negative charges are found on the surface of the membrane, making each rubber particle negatively charged. The negatively-charged rubber particles repel each other, preventing themselves from combining and coagulating.
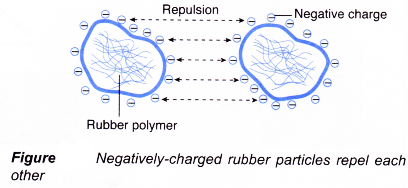
- Acids such as methanoic acid (forfnic acid) are added to make the latex coagulate.
- Hydrogen ions from the acid neutralise the negative charges on the surface of the membrane. A neutral rubber particle is formed.
- When these neutral particles collide with each other, their outer membrane layers break up. The rubber polymers are set free.
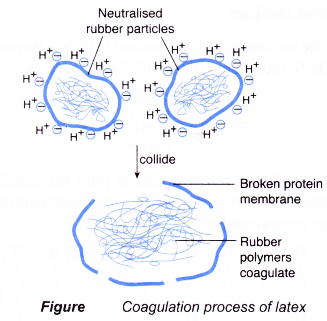
- The rubber polymers start to coagulate by combining together to form large lumps of rubber polymers which then precipitate out of the latex solution.
- Latex can still coagulate if acids are not added. Normally, the latex will coagulate if left overnight.
- Bacteria from the air slowly attack the protein on the membrane to produce lactic acid. Ionisation of the lactic acid produces hydrogen ions. The hydrogen ions neutralise the negative charges to form neutral rubber particles, allowing coagulation to occur.
- Alkalis such as ammonia solution are added to latex to prevent coagulation.
- The hydroxide ions from alkali neutralise hydrogen ions produced by lactic acid as a result of bacterial attack on protein.
- Because there are no hydrogen ions to neutralise the negative charges on the rubber particles, they remain negatively charged and hence cannot combine and coagulate.
Coagulation of latex experiment
Aim: To investigate the coagulation of latex.
Materials: Latex, 2 mol dm-3 ethanoic acid, 2 mol dm-3 ammonia solution, red and blue litmus paper.
Apparatus: Beakers, dropper, glass rod.
Procedure:
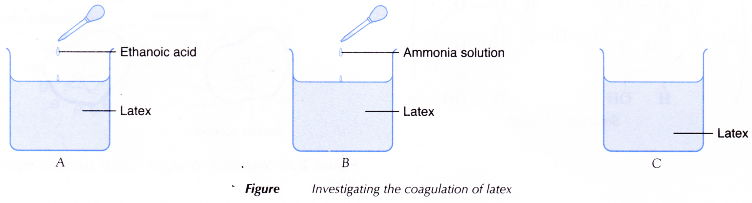
- About 20 cm of latex is poured into each of the three beakers labelled A, B and C.
- Ethanoic acid is added drop by drop into beaker A until the latex becomes acidic (blue litmus paper turns red). The mixture is stirred after each addition of acid.
- Ammonia solution is added drop by drop into beaker B until the latex becomes alkaline (red litmus paper turns blue). The mixture is stirred after each addition of alkali.
- The three beakers are left overnight. Any changes that occur are noted.
Observations:
| Beaker | Observation |
| A | The latex coagulates very quickly. |
| B | The latex does not coagulate. |
| C | The latex coagulates slowly. |
Discussion:
- Ethanoic acid is an organic acid. It ionises in water to produce hydrogen ions. The positive hydrogen ions help to neutralise the negatively-charged rubber particles, allowing the latex to coagulate.
- Ammonia solution neutralises lactic acid secreted by bacteria when they attack the protein membrane. This keeps the rubber particles negatively charged and prevents coagulation.
- When latex is exposed to air, bacteria from the air slowly attack the protein membrane to produce lactic acid. The lactic acid helps to coagulate the latex.
Conclusion:
Acids speed up the coagulation of latex, whereas alkalis prevent the coagulation of latex.
People also ask
- What are carbon compounds?
- Chemical Properties of Carbon Compounds
- How are alkanes formed?
- What is an alkene in chemistry?
- What is an isomerism?
- What is alcohol and how is it made?
- How are carboxylic acids formed?
- How esters are formed?
- What are fats and oils?
- How palm oil is extracted?
- Order in Homologous Series
- What is the monomer of natural rubber?
- Classification of Hydrocarbons
- What is the homologous series of hydrocarbons?
- Properties and Uses of Ethanol
- Properties and Uses of Ethanoic Acid
