How Acid rain is formed equations?
Formation of acid rain:
- Sulphur dioxide accounts for most of the acid rain problems.
(a) When sulphur dioxide dissolves in water, sulphurous acid is formed.
SO2(g) + H2O(l) → H2SO3(aq)
(b) Sulphur dioxide can react with oxygen and water to form sulphuric acid.
2SO2(g) + O2(g) + 2H2O(I) → 2H2SO4(aq) - Acid rain occurs when the pH of rain water falls between 2.0 to 5.5. This is due to the presence of sulphurous acid, sulphuric acid and nitric acid in rain water.
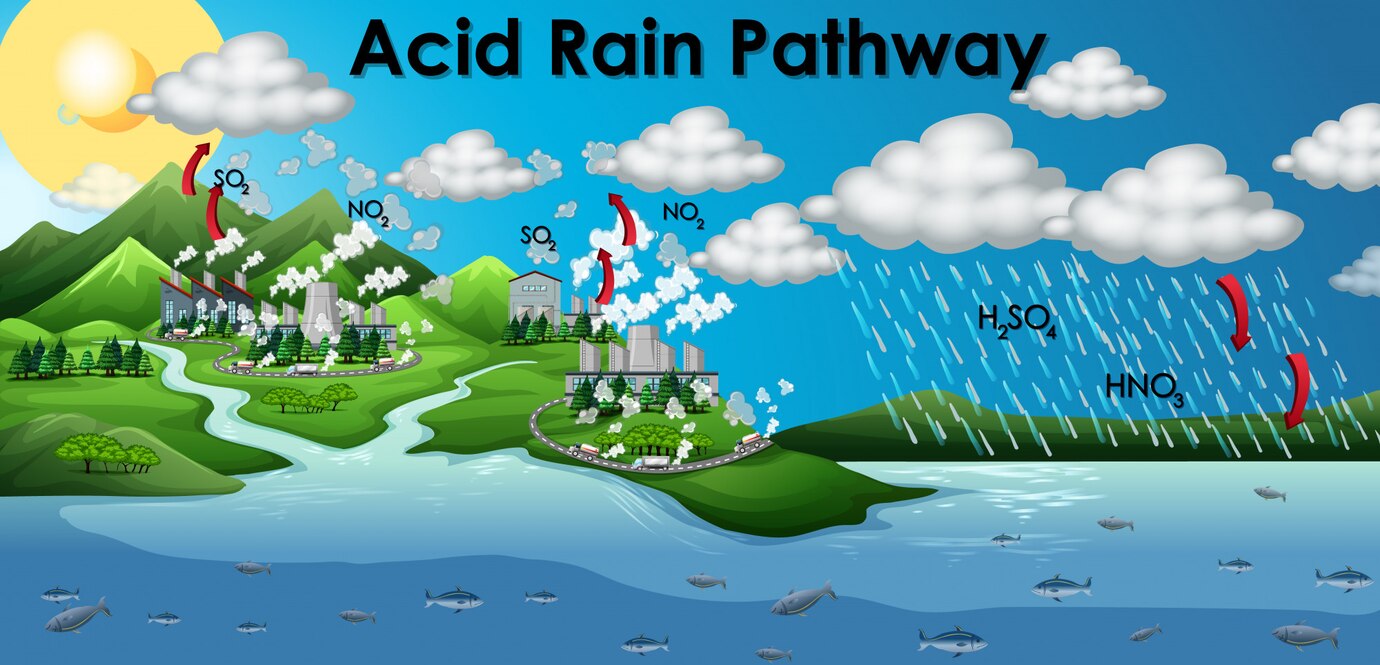
Sources of SO2
- Almost all the sulphur dioxide in the air comes from
(a) vehicles (vehicles release SO2 by burning of fossil fuels)
(b) industrial sources (SO2 released from the factories causes air pollution) - Other sources that release sulphur dioxide in the atmosphere are:
(a) Volcanic eruption
(b) Extraction of metals from their sulphide ores
(c) Burning of fossil fuels such as coal and petroleum with high sulphur content
(d) Burning of waste products manufactured from sulphuric acid such as rayon
People also ask
- How is Sulfuric Acid Made?
- Uses of sulphuric acid in daily life
- What is the Haber process used for?
- What are the physical properties of ammonia?
- Uses of ammonia in our daily life
Effects of Acid Rain on the Environment
Air pollution
- Acid rain pollutes the air and corrodes buildings, monuments and statues made of metals and marble.
- Metals like iron and calcium carbonate react with the acid in the rain slowly as follows:
Fe(s) + H2SO4(aq) FeSO4(aq) + H2(g)
CaCO3(s) + H2SO4(aq) CaSO4(s) + CO2(g) + H2O(l)
Water pollution
- Acid rain increases the acidity in rivers and lakes.
- The acidic water kills aquatic and organisms like planktons and fishes, disturbing ecosystem.
Soil pollution
Acid rain:
- (a) increases the acidity of soil
The reaction of sulphuric acid with aluminium compounds forms aluminium sulphate which can damage the roots of trees. The damage roots are easily attacked by viruses and bacteria. - (b) leaches minerals and nutrients in the soil
The acid reacts with the minerals in the soil forming soluble salts and they are carried away by rain water. - (c) destroys plants and trees in the forests
Apart from the acidic soil, the trees die of combination of malnutrition and diseases.
How can we reduce the effects of acid rain?
Ways to control the effects of acid rain:
- Use low-sulphur fuels to reduce the amount of sulphur dioxide released into the air.
- Remove sulphur dioxide from the waste gas before it is emitted into the atmosphere.
(a) Powdered limestone is blown into the combustion chamber. It decomposes into calcium oxide.
CaCO3(s) CaO(s) + CO2(g)
(b) Calcium oxide reacts with sulphur dioxide to form calcium sulphite.
CaO(s) + SO2(g) CaSO3(s)
(c) Calcium sulphite is then oxidised to calcium sulphate to be used in the building industry. - Neutralise the acidic soil and water by treating them with calcium oxide (lime), calcium hydroxide and calcium carbonate.
